Small Scale Expression of Tagged Recombinant Proteins

Small Scale Expression of Tagged Recombinant Proteins
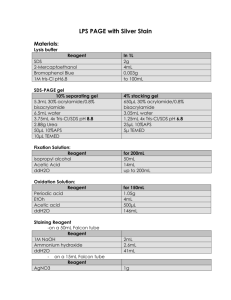
Lysis buffer
Reagent
SDS
2-Mercaptoethanol
Bromophenol Blue
In 100mL
2g
4mL
0.003g
1M tris-Cl pH6.8
SDS-PAGE gel
10% separating gel
5.3mL 30% acrylamide/0.8% bisacrylamide
6.5mL water
3.75mL 4x Tris-Cl/SDS pH 8.8
50μL 10%APS
10μL TEMED
Electroblotting buffer:
Reagent
Tris Base
Glycine
Methanol ddH
2
0
Blocking Solution:
In 4L
9.664g
45.04g
800mL
To 4L
Reagent
Roche Blocking Reagent (10X)
PBS
1XPBS-AT (PBS-Azide/Tween):
10XPBS
Reagent
NaN
3
Tween20 ddH
2
0
In 1L
100mL
0.20g
0.5mL
To 1L
Store @4°C, no need to be sterile
Wash solution (0.5% blocking solution): to 100mL
4% stacking gel
650μL 30% acrylamide/0.8% bisacrylamide
3.05mL water
1.25mL 4x Tris-Cl/SDS pH 6.8
25μL 10%APS
5μ TEMED for 50mL
5mL
45mL
In 5L
12.08g
56.3g
1000mL
To 5L
In 2L
200mL
0.40g
1mL
To 2L
Reagent
Roche Blocking Reagent (10X)
PBS
2.5mL
47.5mL
Alkaline phosphatase conjugated antibody solution (on 0.5% blocking solution)
Reagent for 10mL
Roche Blocking Reagent (10X)
Antibody
-Goat anti-mouse IgM HRP (1:2000)
PBS
500μL
5μL
10mL
Methods: a.
Transformation of BL-21 (Novagen Singles) competent cells with recombinant plasmid:
1.
Thaw the required number of tubes of cells on ice and mix gently to ensure that the cells are evenly suspended.
Transform also with empty vector.
2.
Add 1 μl of the DNA solution directly to the cells. Stir gently to mix.
3.
Place the tubes on ice for 5 min.
4.
Heat the tubes for exactly 30 s in a 42°C water bath; do not shake.
5.
Place on ice for 2 min.
6.
Add 250 μl of room temperature SOC Medium to each tube.
7.
Selection for transformants is accomplished by plating on LB media containing 100µg/mL Carbenicillin or Ampicillin b.
Protein Expression
1.
Select 3 colonies/ recombinant plasmid and one for empty vector. Grow 5ml cultures of LB with 100µg/mL Carbenicillin or
Ampicillin to an A600 of 0.8 (3-5h) with vigorous agitation at
37ºC. Store 2.5mL of this cells as un-induced sample (store in ice).
2.
Induce fusion protein expression by adding 3µL of 100mM
IPTG(final concentration of 0.1mM). A higher concentration might be used (up to 1mM).
3.
Continue incubation for an additional 1-2h.
4.
Spin down induced and non-induced cells.
5.
Store 1ml supernatant at -20°C.
6.
Resuspend pellet on 100µL of lysis buffer with 2-
Mercaptoethanol, boil for 5-10 minutes before loading. c.
Making the gel:
1.
Install equipment.
2.
Mix the reagents for the separating gel.
3.
Pour separating gel, cover with a layer of ddH2O; wait for 45 mins or until gel polymerizes. Take out water layer.
4.
Mix reagents for stacking gel, and pour. Avoid bubbles.
5.
Insert comb. Allow to polymerize for 45 mins or until gel polymerizes.
6.
Install gel in the equipment. Add 1x SDS running buffer.
7.
Load 6μL of Kaleidoskope Marker, and 10µL of boiled sample.
Run at 35mAmp for 2h. d.
Coomassie Stain (Bio-Rad Bio-Safe Coomassie)
1.
Wash the gel(s) 3 times for 5 minutes in 200mL ddH2O.
2.
Remove all water and add 50mL (or enough to cover the gel) of Coomassie stain. Gently shake for 20mins-1h.
3.
Rinse gel in 200mL ddH2O for at least 30 minutes. Stained gels can be stored in water. e.
Transfer:
1.
Cut the membrane and filters, while wearing gloves!!! Soak membrane, filters, and fiber pads in transfer buffers for about
15 minutes)
2.
Prepare gel sandwich: a.
place the cassette with the black side down, on a clean surface. b.
place one of the fiber pads on the black side. c.
place a sheet of filter paper on the fiber pad. d.
place the gel on the filter paper. e.
place the pre-wet membrane on the gel. f.
place the second sheet of filter paper on top of the membrane g.
add the last fiber pad. h.
remove bubbles
3.
Place the sandwich on the electrode cell, and add transfer buffer until full.
4.
Place cell on ice box, add ice.
5.
Connect to power supply, and run for 1.5h at 1 amp.
6.
Block membrane. f.
Detection of proteins:
1.
Block the membrane overnight at 4°C without agitation (or for 1h at room temp. with agitation) using 1X blocking solution in PBS.
2.
After blocking, incubate in its respective conjugated antibody diluted as required (in 0.5% blocking solution in PBS).
Agitate for 1h at room temp.
3.
Decant antibody (can reuse if stored at -20°C).
4.
Wash 3 times on 1XPBS-AT for 1 minute, and 2x 5minutes minutes each time.
5.
Decant all washing buffer. Add 2-3 mL (until covering membrane) of the BCIP/NBT Phosphate substrate to the membrane.
6.
To stop staining, decant all substrate and wash profusely with ddH2O.