Supplementary Information (doc 44K)
advertisement

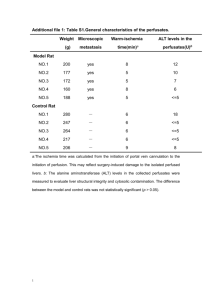
Supplemental methods Rats All rats were housed in individual cages with a 12/12 light dark schedule (lights on at 7.00 A.M.). Standard Rodent Chow and water were available ad libitum, unless stated otherwise. All procedures were approved by the animal care committee of the Royal Netherlands Academy of Arts and Sciences. Tail incision In the second week after arriving in the facility (week 0) a baseline blood sample was taken. All blood samples in week 0 to 4 were taken by tail incision (1). On the days of blood sampling, the rats were fasted from 09.00 A.M. and a blood sample was taken ~4 hours later between 1.00 and 2.00 P.M. Denervation and jugular vein surgery For both surgeries anaesthesia with 0.8 ml/kg Hypnorm i.m. (Janssen, High Wycombe, Buckinghamshire, UK) and 0.4 ml/kg Dormicum s.c. (Roche, Almere, The Netherlands) was used. One day after the baseline blood sampling in week 0, rats received a sham, selective parasympathetic or sympathetic denervation (2). To achieve a hepatic sympathectomy (Sx), nerve bundles running along the hepatic artery proper were transected using microsurgical instruments. Any connective tissue attachments between the hepatic artery, bile duct and portal vein were also dissected. To achieve a hepatic parasympathectomy (Px), the common hepatic vagal branch was transected by stretching the fascia containing the common hepatic vagal branch and transecting neural tissue between the ventral vagal trunk and liver. Sham operated rats underwent all procedures as described above, except for transection of the neural tissue. The day after the tail vein blood sampling in week 4, the rats were fitted with an intra-atrial silicone cannula into the right jugular vein according to the method of Steffens (3). The cannula was tunneled to the head subcutaneously, fixed with dental cement to four stainless steel screws inserted into the skull. A mixture of 60% amoxicillin, 20% heparin, and 20% saline in polyvinylpyruvidon (PVP;Sigma) was used to fill the cannulas and prevent inflammation and occlusion. Measurement of VLDL-TG secretion In week 5 rats were connected to an infusion swivel (Instech Laboratories, PA, USA) on the day before the actual experiment, for adaption. One gram Tyloxapol (Triton WR 1339; Sigma-Aldrich, Germany) was dissolved in 5.7 ml 0.9% saline overnight on a shaker. On the next day rats were fasted from 09.00 A.M. onwards and external lines for blood sampling were connected. At 1.00 P.M. a baseline blood sample from the jugular vein was taken and an intravenous dosage of 0.7 ml Tyloxapol in saline was given. At 20-min intervals blood samples were drawn from the jugular vein catheter during 100 minutes. Tyloxapol inhibits lipoprotein lipase, thereby blocking the uptake of triglycerides by the peripheral tissues. In the absence of chylomicrons carrying triglycerides from the gut, the increase in plasma triglycerides reflects VLDL-TG secretion (4). The VLDL-TG secretion (mmol/l/h) was determined by the slope of the rise in triglycerides over time by linear regression analysis. A linear increase in plasma triglycerides after Tyloxapol was shown by R2 values above 0.98 for all groups. Tissue collection In week 6 rats were fasted from 09.00 A.M. onwards and at 1.00 P.M. a blood sample from the jugular vein was taken. Subsequently, rats were injected with an overdose of pentobarbital IV. Liver tissue was removed and snap frozen for further analysis. When the cannula was blocked, decapitation blood was collected after intraperitoneal injection of pentobarbital. Plasma measurements Glucose concentrations were determined during the experiment in blood spots using a glucose meter (FreestyleTM, Abbott, The Netherlands). Triglycerides and cholesterol were assayed using a kit from Roche (Mannheim, Germany). The WAKO NEFA HR kit (Wako. Chemicals, Neuss, Germany) was used to measure FFA in plasma. By using radioimmunoassay kits, plasma insulin (LINCO Research, St. Charles, MO, USA) and corticosterone (ICN Biomedicals, Costa Mesa, CA) were measured. Liver measurements The effectiveness of the hepatic sympathetic denervation was checked by measurement of liver noradrenaline concentration using an in-house HPLC method with fluorescence detection. Liver tissue samples of 50 mg were homogenized in 1 ml of ice-cold NH4Cl buffer (0.2 M, containing 12 nmol/l -methylnorepinephrine and 1 g/l EDTA, pH 7.0) and centrifuged twice (14,000 rpm) for 15 min at 4°C. Essentially, noradrenaline was selectively isolated by liquid-liquid extraction (5) and derivatized with the fluorescent 1,2-diphenylethylenediamine (6). The fluorescent derivatives were separated by reversed-phase liquid chromatography and detected by fluorescence detection (510 pump, 717 plus autosampler, and 474 fluorescence detector; Waters Chromatography). Separation of noradrenaline from other endogenous compounds was achieved with a Waters Xterra RP18 column (5 m, 3.9 x150 mm). As an internal standard, -methylnorepinephrine was used. The detection limit for noradrenaline was 0.05 nmol/l. All obese fa/fa Sx rats included in the experiment showed liver noradrenaline concentrations below 1 ng/g (0,59±0,07; mean±SEM) compared to obese fa/fa sham rats (18,41±4,03; mean±SEM). Three obese fa/fa Sx rats were excluded based on higher liver noradrenaline concentrations. Triglycerides in 50-100 mg liver tissue were measured after a single step lipid extraction with methanol and chloroform (ratio 1:3) in a tissue-to-solvent ration of 1:80. (7). The pellets were finally dissolved in 2 % Triton X-100 (Sigma-Aldrich, Germany) and triglycerides were measured using “Trig/GB” kit (Roche, Mannheim, Germany). Liver acylcarnitines levels in 60 mg of liver tissue were measured essentially as described previously (8). Statistical analysis Data are presented as mean ± SEM. For every experiment, two separate analyses were performed. First, Fa/fa sham and fa/fa sham rats were compared by a repeated measurements general linear model (GLM) for comparing outcome measures at multiple time points (with Genotype as between-animal factor and Time as withinanimal factor) or a t-test for single outcome measures. Second, fa/fa sham, fa/fa Sx and fa/fa Px rats were compared by repeated measurements GLM for comparing outcome measures at multiple time points (with Denervation as between-animal factor and Time as within-animal factor) or one-way ANOVA for single outcome measures. A significant (P≤0.05) global effect of repeated measurements GLM or ANOVA was followed by post hoc tests to detect individual group differences (Fisher’s protected least significant difference). Significance was defined at P<0.05. References (1) Fluttert M, Dalm S, Oitzl MS. A refined method for sequential blood sampling by tail incision in rats. Lab Anim. 2000; 34 :372-378. (2) Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci 2004; 24: 7604-7613. (3) Steffens AB. A method for frequent sampling blood and continuous infusion of fluids in the rat without disturbing the animal. Physiology and Behavior 1969; 4: 833-836. (4) Otway S, Robinson DS. The use of a non-ionic detergent (Triton WR 1339) to determine rates of triglyceride entry into the circulation of the rat under different physiological conditions. J Physiol 1967; 190: 321-332. (5) Smedes F, Kraak JC, Poppe H. Simple and fast solvent extraction system for selective and quantitative isolation of adrenaline, noradrenaline and dopamine from plasma and urine. J Chromatogr 1982; 231: 25–39. (6) van der Hoorn FA, Boomsma F, Man in 't Veld AJ, Schalekamp MA. Determination of catecholamines in human plasma by high-performance liquid chromatography: comparison between a new method with fluorescence detection and an established method with electrochemical detection. J Chromatogr 1989; 487: 17-28. (7) Srivastava NK, Pradhan S, Mittal B, Kumar R, Nagana Gowda GA. An improved, single step standardized method of lipid extraction from human skeletal muscle tissue. Analytical Letters 2006; 39: 297-315. (8) van Vlies N, Tian L, Overmars H, Bootsma AH, Kulik W, Wanders RJ, Wood PA, Vaz FM. Characterization of carnitine and fatty acid metabolism in the long-chainacyl-CoA dehydrogenase-deficient mouse. Biochem J. 2005; 387: 185-193.