Figure 5.1 The water molecules consists of 1 oxygen molecule and 2
advertisement

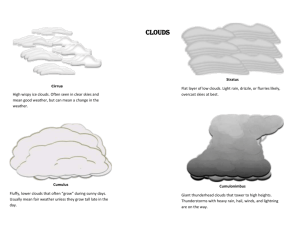
The circles and arrows represent the movement of water molecules. Temperature = 5C Temperature = 15C Figure 4.1 When the air above the water is saturated, the number of molecules condensing equals the number of molecules evaporating from the water. The number of molecules leaving the water increases with increasing temperature. What will happen to the water in the beaker if it is uncovered? (Use other techniques to demonstrate motion other than arrows. Replace circles with a symobl that looks more like a water molecule as in Figure 4.28) Height above the surface (km) 10 8 Tropical Midlatitude summer Artic w inter 6 4 2 0 0 2 4 6 8 10 12 14 16 18 20 Mixing ratio (g/kg) Figure 4.2 The source of atmospheric water vapor comes from the surface of the Earth. Explaining why values of mixing ratio typically decrease with altitude at different regions of the world. Figure 4.3 When the temperature of the web cooled to the dew point temperature, dew formed making the web visible. Figure 4.4 Frost, ice crystal formed by deposition of water vapor on subfreezing surfaces, will form in open fields before before forming under a tree. Notice also the steam fog in this picture. (Might want to label the whitish grass as FROST) The circles represent individual water molecules. Figure 4.5 The smaller the drop the more curved the surface, reducing the number of neighbors for each water molecule at the surface. This curvature effect makes it easier for small drops to evaporate. Figure 4.6 The mean annual number of foggy days with visibility less than 300 m over a poriton of the United States (after Court and Gerston, 1966). Do you know someone who lives in a foggy region of the United States? Figure 4.7 Radiation fog often forms in low lying areas, particularly river valleys. This is a satellite visible image of a radiation fog that occurred in many river valleys through the Appalacian mountains. Figure 4.8 Advection fog is common off the coast of California as warm moist air over the Pacific is advected over the cold coastal waters. (Photograph courtesey of NOAA). Figure 4.9 Steam fog often occurs when cold air moves over warm water. Orographic lifting Convergence of air at the surface solar heating warm air cold air Convection cool air Frontal lifting Figure 4.10 Four mechanisms that cause air to ascend and form a cloud. (Add clouds, see Ahrens Essential Figure 5.8) Cirrus Cirroc um ulus Cum ulonim bus Cirrostratus Altoc um ulus Altostratus Nim bostratus Stratus Stratoc um ulus Cum ulus Figure 4.11 The ten major cloud catagories. (Need a drawing of cloud types with arrows point to pictures. Figure 4.12 Stratus clouds (St) are low altitdue clouds in layers. (I have permission forms and original prints of figures 4.12-4.21, or we can get new ones. Link these figures to Figure 4.11) Figure 4.13 Stratocumulus (Sc) are low-lying clouds. Stratocumulus are distinguished from stratus by variations in color across the sky. Figure 4.14 Nimbostratus (Ns) are deep clouds that bring precipitation and appear dark gray to pale blue in color. Figure 4.15 Cumulus clouds are often observed on a summer day. Figure 4.16 Cumulonimbus clouds are thunderstorm clouds. Figure 4.17 Altostratus clouds (As) are layered clouds that exist in the middle layers of the troposphere. Figure 4.18 Altocumulus (Ac) occur in the middle levels of the atmosphere when the air is moist. Figure 4.19 Cirrocumulus (Cc) are thin, white clouds that appear high in the troposphere. Figure 4.20 Cirrostratus (Cs) are layered clouds that are sometimes observed with a halo. Figure 4.21 Cirrus (Ci) are wispy, fibrous, white clouds that are composed of ice crystals. Plate Needle Column Flat plate Dendrite Figure 4.22 The five basic ice crystal habits are plate, column, dendrite, flat plate and needle. Which shape an ice particle grows as depends on the temperature of the environment it grows. (Need better drafted figures and temperature ranges added.) Figure 4.23 In the solar spectrum clouds tend to cool Earth while in the longwave radiation they tend to warm the planet. (Need a figure that shows clouds reflecting solar radiation back to space and reducing longwave radiation to space, something like Lutgen's figure 3-11 page 57) Figure 4.24 Large water drops fall faster than smaller ones. Because of the differenth fall speeds, water drops collide and coalesce. Figure 4.25 Ice crystals of different sizes or different shapes may collide and stick together. (Need a similar figure to 4.24 but for ice crystals) The circles and arrows represent the movement of water molecules. Ice Super-cooled water Temperature = -5C Figure 4.26 At a given temperature, the saturation vapor pressure over ice is less than the saturation vapor pressure over water. Water molecules in the ice phase are more strongly bonded to each other than when in the liquid phase. More energy is required for the water molecules to escape from the ice phase. (Use other techniques to demonstrate motion other than arrows. Replace circles with a symobl that looks more like a water molecule as in Figure 4.28) Saturation vapor pressure (mb) 1400 6 1200 5 Over water Over ice 4 1000 3 2 1 800 0 -20 600 -15 -10 -5 Temperature (C) 0 400 200 0 -10 Relative humdity less than 100% Relative humdity > 100% 0 10 20 30 40 50 60 70 80 90 100 110 Temperature (C) Figure 4.27 The relationship between temperature and the saturation vapor pressure over water. Conditions along the line represent a relative humidity of 100%. The inset depicts the differences between the saturation vapor pressure over ice and over water. Since the line for water lies above the line representing ice, when the air is saturated (relative humidity equals 100%) with respect to liquid water, it is supersaturated with respect to ice. Movement of water vapor molecules Figure 4.28 The larger saturation vapor pressure over a liquid water surface than over an ice surfaces causes the ice crystal to grow and the super-cooled drops to evaporate. (Show sequence with water drop getting smaller and ice crystal getting larger, see Arhens Essential figure 5.19. Should also use a dendrite shape as Ahrens did.) Figure 4.29 Virga is rain that falls out of the cloud but evaporates before reaching the ground. RAIN SNOW Cloud base Cloud base Temperature below freezing Temperature below freezing Temperature above freezing SNOW Rain Cloud base Temperature below freezing Temperature below freezing Cloud base Temperature below freezing Temperature below freezing Temperature Ice Pellets below freezing (Sleet) Rain freezes on contact FREEZING RAIN SLEET Figure 4.30 The vertical variation of temperature will determine if precipitation falls as rain, freezing rain, sleet or snow. In this example, all the precipitation particles are initially ice crystals. Particle that fall into the melting layer become liquid drops. To form freezing rain or sleet a surface temperature in version is required. (Add more particles and indicate that they are falling.) Figure 4.31 Freezing rain, or an ice storm, occurs when there is a shallow temperature inversion near the ground. Freezing rain covers everything in ice. Making a beautiful, though often treacherious traveling conditions. (Photograph from NOAA). Figure 4.32 Hailstones are the largest form of precipitation. Figure 4.33 Rime forms when a super-cooled fog is present and surface temperatures are below freezing. In this example, rime formed on a spider web. Figure 4.34 As moist air flows over a mountain, clouds form and precipitation falls on the upwind side. Sink motions on the leeward side of the mountain generate a rainshadow. (Need to include parcel with temperatures at 1, 2 and 3 km on each side of the mountain.)