What is Gelation?
advertisement

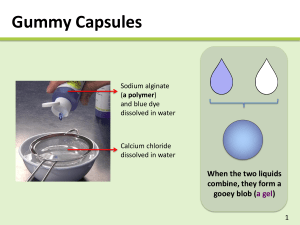
Magic Suspensions Developer: Dr. Mary R. Reidmeyer Project for Grade Level: High School Discipline: Materials, Chemistry Topic Area: Binder Gelation, Crosslinking Time Required: Goals: Demonstrate the ability to form spherical shapes based on the gelation of seaweed derived material. Objectives: Observe the crosslinking of high molecular weight polymer into a gel with exposure to highly charged cations in a solution. Create spherical balls by allowing drops of a ceramic/polymer solution to fall into a soluble salt solution. Discuss what can be created with these ceramic/polymer spheres. Materials: Ammonium alginate powder Talc powder Nepheline Syenite powder Calcium chloride Plastic syringe 12ml with 16 GA blunt needle Beakers 100ml 250ml, 400ml Wire Strainer Stir Bars Magnetic stirrer Balance University of Missouri-Rolla – Ceramic Engineering Department http://campus.umr.edu/ceramics 116092419 Safety Precautions: Follow the instructions. Wear proper safety equipment such as goggles and vinyl gloves. Dispose of the materials properly. Procedure: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Prepare a 1 wt% solution of ammonium alginate. Place a 400ml beaker (with stir bar) on a magnetic stir plate that contains 297g of deionized water. With stirrer creating a moderate vortex, gradually sprinkle the 3g ammonium alginate powder into the water. Do not clump it together. If you do, it will not dissolve in a reasonable amount of time (normally 1530 minutes.) Since the alginate is a dried polymer, first the granules must be dispersed in the water then swelled and disentangled. Prepare a 10wt% solution of calcium chloride. Stir 40g of calcium chloride into 360g of deionized water in a 400ml beaker. Prepare a 25vol% ceramic slurry using the prepared 1 wt% sodium alginate solution. To 75ml of ammonium alginate solution stir in 15g of nepheline syenite and 40g of talc powder. Stir thoroughly for several minutes until the slurry becomes smooth. Place the calcium chloride solution on the magnetic stir and set to stir very slowly. Take the syringe and draw up approximately 6ml of 1wt% sodium alginate solution. Hold the syringe over the beaker containing the calcium chloride solution. Gently press the syringe plunger so that drops of solution are formed on the tip of needle. Allow the drops to fall into the calcium chloride solution. After several dozen transparent spheres of alginate can be observed in the calcium chloride beaker cease dropping solution. Allow the spheres to circulate in the beaker for several minutes. Place the wire strainer over the top of a clean 400ml beaker and pour the sphere containing solution through the strainer. Retain the calcium chloride solution for future use. Gently rinse the spheres in the strainer with deionized water. Rinse some out of the strainer into a glass dish for observation. Examine others with your fingers and determine their properties. Set your calcium chloride solution back on the magnetic stirrer. Squirt the remaining alginate solution in the syringe into a waste container. Stir the ceramic slurry again to homogenize it. Take the syringe and draw up approximately 6ml of ceramic slurry. Hold the syringe over the beaker containing the calcium chloride solution. Gently press the syringe plunger so that drops of slurry are formed on the tip of the needle. Allow the drops to fall into the calcium chloride solution. University of Missouri-Rolla – Ceramic Engineering Department http://campus.umr.edu/ceramics 116092419 11. 12. After dozens of ceramic spheres are created, allow them set a few minutes then strain and wash them as done previously with the alginate sphere. Examine the ceramic spheres. Observations and Questions: 1. 2. 3. 4. 5. 6. 7. Describe what happens when high molecular weight ionic polymer is exposed to highly charged cations. How would potassium chloride or magnesium chloride solutions interact with the sodium alginate solution? What would happen if you accidentally stirred the alginate solution with the spatula (dirty) you used to stir the calcium chloride solution? (Try pouring a small amount of each solution together in another beaker.) How would the alginate solution behave if was mixed with a less soluble salt such as calcium sulfate. (Hint: Calcium chloride salt is highly soluble, calcium sulfate is slightly soluble.) What variables would you control to change the size of the spheres created? What would happen if you extruded a column of alginate solution into the calcium chloride solution? What are some practical uses of this gelation process? (Think about dentistry, foods, cosmetics, etc.) Teacher’s Note: Supplemental MaterialVocabulary: Alginate- A salt of alginic acid, such as sodium or ammonium alginate. Alginic acid- An insoluble colloidal acid in the form of a polysaccharide that is abundant in the cell walls of brown algae, kelp. Crosslink- an atom or group that connects parallel chains in a complex chemical molecule. Gel- a colloidal mixture of solid and liquid of jelly-like consistency. Molecular weight- The sum of the atomic weights of all the atoms in a molecule. Also called formula weight. University of Missouri-Rolla – Ceramic Engineering Department http://campus.umr.edu/ceramics 116092419 Nepheline syenite- K2O·Na2O·4Al2O3·9SiO2; hardness (Mohs) 6; sp. gr. 2.60; an igneous rock consisting of a mixture of potash feldspar and soda feldspar used in ceramics to lower the firing temperature. Salt- A chemical compound formed by replacing all or part of the hydrogen ions of an acid with metal ions or electropositive radicals. Slurry- A thin mixture of a liquid, especially water, and any of several finely divided substances, such as cement, plaster of Paris, or clay particles. Solution- A homogeneous mixture of two or more substances, which may be solids, liquids, gases, or a combination of these. Talc- 3MgO·4SiO2·H2O; m.p. above 1400°C; sp. gr. 2.7; hardness (Mohs) 1- 1.5; a natural hydrous magnesium silicate commonly used in low fire pottery bodies. Background Information: Gums and ResinsMany different materials are used as additives in the processing of ceramic materials. Polymer molecules are adsorbed between ceramic particles and can provide a temporary bond between the particles to allow molding or forming of articles. Depending on the nature of the organic additive it can burn cleanly way during heating or leave behind inorganic components which can react with the particles. Many organic binders such as cellulose, gums, polysaccharides (starch), lignin, alginates, glycols, polymerized alcohols and acrylic resins burn away during the heating process before ceramic bonds are formed. These binders can be purchased in different molecular weights and grades. Some of the favorite additives are also used extensively in the food industry as thickening or emulsion controlling additives. The salts of alginic acid, such as ammonium alginate and sodium alginate, are derived from an extract of Kelp seaweed. The empirical formula for sodium alginate is C6H7NaO6. Algin polymers will react with most polyvalent cations (except magnesium) to form crosslinkages. As the concentration of polyvalent cations increase, the algin solution, thickens, gels, and can even precipitate. Calcium is one of the most common polyvalent cations used to gel alginates. Highly soluble calcium compounds can be used to from insoluble filaments, films and spheres. Calcium alginate is not water soluble. Slowly dissolving calcium compounds can gradually thicken a solution. Fortunately alginates in solution are compatible with a wide variety of materials including other thickeners, synthetic resins, sugars, oils, fats, waxes, pigments and various surfactants. Because of this we can create gels and insoluble filaments that contain a wide variety of materials. University of Missouri-Rolla – Ceramic Engineering Department http://campus.umr.edu/ceramics 116092419 What is Gelation? Gelation occurs in solutions that contain long chain molecules when something causes the chains to interact. When the long chain molecules interact, they produce a threedimensional structure. Commonly this interaction is cause by a thermal or chemical change. Depending on the nature of that interaction the gelation may be reversible or irreversible. In a sodium alginate solution when calcium cations are introduced into the solution, a chemical change occurs; Nan alginate(sol) + n/2 Ca2+(solution) → n Na+(solution) + Can/2 alginategel) This chemical gelation is practically irreversible. An example of reversible gelation is a methyl cellulose solution. Upon heating the cellulose molecules in the solution dehydrate and three dimensional bonds form creating a gel. Upon cooling the cellulose molecules rehydrate, the bonds are broken and the gelation disappears. Forming MethodsA great many forming methods are used to create useful objects from powdered materials. For ceramics, binders help hold the powder particles together in the desired shape until heat treatment forms a permanent strong bond. The method used depends on the objects size, geometry, surface finish, required tolerances and the productivity required. Powders can be pressed into shapes but these shapes relatively simple. Objects can be cast from powder/water suspensions into porous multipart molds to produce more complex shapes. This method while very effective is labor intensive. Soft plastic mixtures of powders and binders can be extruded like pasta and simple cross-sectional objects can be produced in large quantities. Similar mixtures can be injection molded like a plastic. While these methods can form greater quantities or more complex objects, the amount of binder required can affect the ability to produce these easily and quickly. Many creative methods are developed and used by material engineers and scientists. The gelling of alginate solutions containing powder is just one example of these creative forming methods. University of Missouri-Rolla – Ceramic Engineering Department http://campus.umr.edu/ceramics 116092419