Depo-Medrone - The British Society for Rheumatology
advertisement
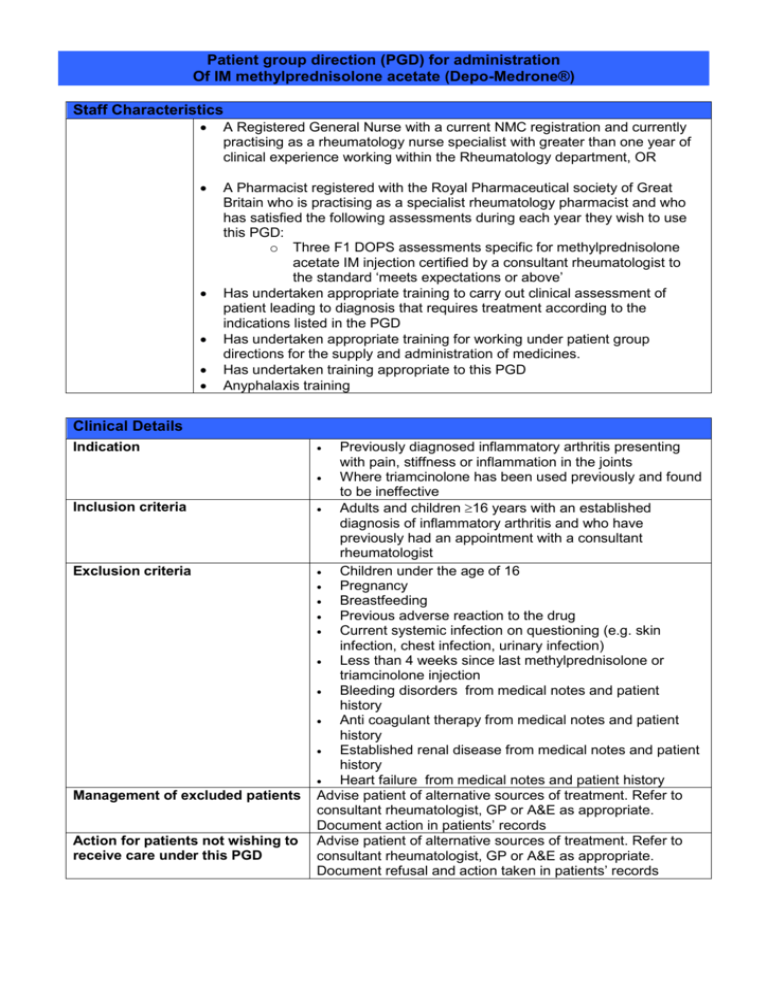
Patient group direction (PGD) for administration Of IM methylprednisolone acetate (Depo-Medrone®) Staff Characteristics A Registered General Nurse with a current NMC registration and currently practising as a rheumatology nurse specialist with greater than one year of clinical experience working within the Rheumatology department, OR A Pharmacist registered with the Royal Pharmaceutical society of Great Britain who is practising as a specialist rheumatology pharmacist and who has satisfied the following assessments during each year they wish to use this PGD: o Three F1 DOPS assessments specific for methylprednisolone acetate IM injection certified by a consultant rheumatologist to the standard ‘meets expectations or above’ Has undertaken appropriate training to carry out clinical assessment of patient leading to diagnosis that requires treatment according to the indications listed in the PGD Has undertaken appropriate training for working under patient group directions for the supply and administration of medicines. Has undertaken training appropriate to this PGD Anyphalaxis training Clinical Details Indication Inclusion criteria Exclusion criteria Management of excluded patients Action for patients not wishing to receive care under this PGD Previously diagnosed inflammatory arthritis presenting with pain, stiffness or inflammation in the joints Where triamcinolone has been used previously and found to be ineffective Adults and children 16 years with an established diagnosis of inflammatory arthritis and who have previously had an appointment with a consultant rheumatologist Children under the age of 16 Pregnancy Breastfeeding Previous adverse reaction to the drug Current systemic infection on questioning (e.g. skin infection, chest infection, urinary infection) Less than 4 weeks since last methylprednisolone or triamcinolone injection Bleeding disorders from medical notes and patient history Anti coagulant therapy from medical notes and patient history Established renal disease from medical notes and patient history Heart failure from medical notes and patient history Advise patient of alternative sources of treatment. Refer to consultant rheumatologist, GP or A&E as appropriate. Document action in patients’ records Advise patient of alternative sources of treatment. Refer to consultant rheumatologist, GP or A&E as appropriate. Document refusal and action taken in patients’ records Drug details Name, composition, form and strength of medicine Legal classification Route Dosage Frequency Duration of treatment Maximum or minimum treatment period Quantity to administer Cautions Side effects Advice to patient Methylprednisolone Acetate BP 40 mg/ml injection in type I flint glass vials of 1, 2 and 3ml POM Intra muscular Adults only: <50kgs (8st) 80mgs >50kgs (8st) 120mgs At least 4 weeks between injections. No more than 4 injections a year Single dose Single dose Single dose As per data sheet Facial flushing Alteration in glycaemic control (relevant to diabetes) Subcutaneous atrophy/skin depigmentation at injection site Small risk of infection at injection site (1:10,000) Anaphylactic reaction Many other adverse effects listed in SPC but are not applicable following a single dose Discuss potential side effects Product information sheet/ARC leaflet offered to the patient If diabetic, inform patient that blood glucose levels may be elevated for upto one week following injection. If persisting elevation, patient should discuss with GP In the event of a serious or persistent adverse effect the patient will be advised to contact their GP / A&E or the rheumatology department Records and Follow up Records/audit trail Patients’ name, address, date of birth and GP Diagnosis Dose and form administered Advice given to patient Product and expiry date Name of person who administered medication Details of any adverse drug reaction and actions taken Documentation in the patients medical record Appointment in rheumatology follow up clinic Follow up Managerial content of patient group direction for IM methylprednisolone acetate (Depo-Medrone®) This patient group direction must be agreed to and signed by all health care professionals involved in its use. The Trust PGD chairperson should hold the original signed copy. The PGD must be easily accessible in the clinical setting. Organisation Brighton and Sussex University hospitals NHS Trust Authorisation Lead Pharmacist Name: Position: Date: Lead Consultant Signature: Name: Position: Signature: Name: Position: Date: Lead Nurse Signature: Name: Position: Date: Signature: Name: Position: Date: Signature: Name: Position: Date: Signature: Date: Organisational authorisation by: Date for review:











