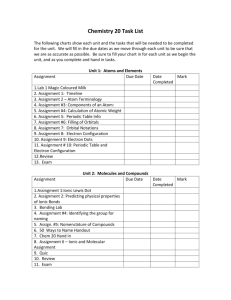
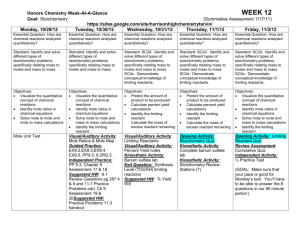
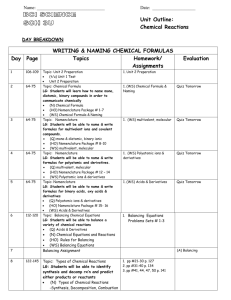
Unit 5: Chemical Bonding and Nomenclature
advertisement

PAP/SPAN Chemistry Agenda Unit: Quantitative Composition of Chemical Reactions Introduction: Real chemistry begins with an understanding of chemical composition; what is the molecular make-up of chemicals and how do they react. In this unit, you will learn how to represent chemical reactions using balanced equations to determine quantitative information about reactivity. Learning Targets: 1. I can write a balanced equation using the correct nomenclature and the Law of Conservation of Mass 2. I can calculate mole relationships including: molar mass, mole fraction, empirical and molecular formulas and molarity 3. I can identify the types of chemical reactions and calculate values using stoichiometry 4. I can identify the limiting reactant and the related calculations. Critical Vocabulary: 6-9 Percent composition Mole Avogadro’s number Empirical formula Molecular formula Reactant Tentative Schedule Assignment Read Chapter 6 & 8: Nomenclature/Equations Assignment: Nomenclature packet Balancing Equations and Nomenclature Discussion Periodic Properties Lab Post Lab discussion and Practice Problems Nomenclature and Balancing Quiz Read Chapter 7: Quantitative Composition Assignment: pg. 145-146 Review Problems 1-10 Mole Concept Discussion and Practice Simple v/s True Activity Mole Concept and Molarity Cumulative Quiz: Nomenclature, Balancing and Mole Concept Review Chapter 8: Equations Assignment: pg. 171-172 Review problems 1-8; Paired Exercises 1-4, 8-9 Simple Chemical Reactions Post Lab Discussion and Practice Balancing Equations and Stoichiometry Cumulative Quiz: Nomenclature, Equations and Balancing, Mole Concept Read Chapter 9: Stoichiometry Assignment: pg. 193 Review Problems 1-6; Paired exercises 2, 4, 6 Stoichiometry Limiting Reactant Cumulative Quiz: Nomenclature, Product Endothermic Exothermic Molar mass Stoichiometry Mole ratio Method of Instruction Limiting reactant Theoretical yield Actual yield Percent yield Practice Due Date 2/1 Can Be Done at Home Yes Grading Lecture & Practice -- No --- Lab Day 2/1 Research & Practice 2/2 No Lab 2/2 Yes Homework Quiz Research & Questions 2/3 2/6 No Yes Assessment Homework Lecture & Calculations Inquiry Calculations Quiz --2/9 2/10 2/10 No Yes Yes No --Lab Homework Assessment Research & Practice 2/13 Yes Homework Lab Day 2/14 2/15 No Lab Lecture & Practice Practice Problems Quiz --2/17 2/17 Yes Yes No --Homework Assessment Research & Practice 2/21 Yes Homework Inquiry Lab Quiz 2/23 2/24 2/24 Yes No No Lab Lab Assessment Homework Equations and Balancing, Mole Concept and Stoichiometry Review Chapter 9: Stoichiometry Assignment: pg. 194 -197 #9, 11-13, 18, 24, 40-41 Quantitative Chemistry Exam Research & Practice 2/27 Yes Homework Summative Exam 2/29 No Assessment Additional Materials may be assigned periodically throughout the unit Schedule & dates are subject to change according to teacher discretion. Assignments are due at the beginning of class on the DUE DATE. All Late work will be penalized 10% each day it is late and will not be accepted after 5 school days. Make sure you submit assignments worth points by the DUE date. Proper attire must be worn on lab days.