Chemical Equations
advertisement

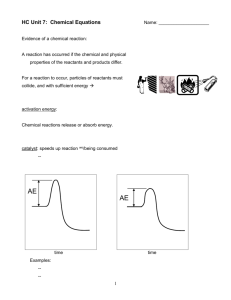
Unit 7: Chemical Equations Name: ____________________ Evidence of a chemical reaction: A reaction has occurred if the chemical and physical properties of the reactants and products differ. For a reaction to occur, particles of reactants must collide, and with sufficient energy activation energy: Chemical reactions release or absorb energy. catalyst: speeds up reaction wo/being consumed … AE AE time time Examples: 1 Factors that influence the rate of a reaction To make reaction rate increase… concentration of reactants particle size temperature mechanical mixing pressure catalyst nature of reactants Balancing Chemical Equations In a reaction: To balance, modify only _____________. Changing a ___________ changes the substance. Right now, _______________ don’t enter into our “balancing” picture. SiCl4 + H2O SiO2 + Rule of Thumb A: HCl Au2S3 + V2O5 + HCl VOCl3 + H2 Au + H2S Rule of Thumb B: H2O Hg(OH)2 + 2 H3PO4 Hg3(PO4)2 + H2O CaC2 + H2O C2H2 + CaSi2 + SbI3 Si + CH3OH Al + CaO Sb + Al(CH3O)3 + C2H2 + O2 CO2 + H2O ** C3H8 + O2 CO2 + H2O ** C5H12 + O2 CO2 + H2O ** CaI2 H2 ** = Reaction Conditions and Terminology Certain symbols give more info about a reaction. (s) = (l) = (g) = (aq) = More on aqueous… -- “soluble” or “in solution” also indicate that a substance is dissolved in water -- acids are aqueous solutions Other symbols… means… means _____ is added to the reaction. Temp. at which we perform rxn. might be given. The catalyst used might be given. precipitate: a solid product that forms in an aqueous solution reaction 3 Word Equations word equation: Solid iron reacts with oxygen gas to yield solid iron(III) oxide. skeletal equation: balanced equation: Write a balanced equation (w/rxn conditions) from the following word equations. EX. Solid sodium reacts w/oxygen to form solid sodium oxide. EX. Aqueous aluminum sulfate reacts w/aqueous calcium chloride to form a white precipitate of calcium sulfate. The other compound remains in solution. EX. Methane gas (CH4) reacts with oxygen to form carbon dioxide gas and water vapor. EX. Write equations for the combustion of C7H16 and C8H18. 4 Classifying Reactions four types A. synthesis: simpler substances combine to form more complex substances sodium + chlorine gas sodium chloride B. decomposition: complex substances are broken down into simpler ones lithium chlorate lithium chloride + oxygen C. single-replacement: one element replaces another aluminum + copper(II) sulfate ? D. double-replacement: lead(IV) nitrate + calcium oxide ? How do we know if a reaction will occur? For single-replacement reactions, Ba + FeSO4 use Activity Series. In general, Mg + Cr(ClO3)3 elements above replace elements below. Pb + Al2O3 NaBr + Cl2 FeCl3 + I2 CoBr2 + F2 For double-replacement reactions, reaction will occur if any product is: Pb(NO3)2(aq) + KOH(aq) + FeCl3(aq) + KI(aq) H2SO4(aq) Cu(NO3)2(aq) 5 more specifically… Ions in Aqueous Solution Pb(NO3)2(s) Pb(NO3)2(aq) NO3– NO3– Pb2+ Pb2+ NO3– NO3– dissociation: NaI(s) Na+ NaI(aq) I– Na+ I– Mix them and get the overall ionic equation… Cancel spectator ions to get net ionic equation… Mix together Zn(NO3)2(aq) and Ba(OH)2(aq): Zn(NO3)2(aq) Ba(OH)2(aq) NO3– OH– Zn2+ Ba2+ NO3– OH– Mix them and get the overall ionic equation… Cancel spectator ions to get net ionic equation… 6 Polymers and Monomers polymer: a large m’cule (often a chain) made of many smaller m’cules called monomers. Polymers can be made more rigid if the chains are linked together by way of a cross-linking agent. Monomer Polymer amino acids……………………………….. nucleotides (w/N-bases A,G,C,T/U)…….. styrene……………………………………... PVA………………………………………… Quantitative Relationships in Chemical Equations 4 Na(s) + O2(g) 2 Na2O(s) ** Particles Moles Grams SUBSTANCE “B” SUBSTANCE “A” Mass Use coeff. from bal. eq. to go from “moles of A” to “moles of B” Vol. MOL MOL EX. 4 Na(s) + O2(g) 2 Na2O(s) How many moles oxygen will react with 16.8 moles sodium? EX. How many moles sodium oxide are produced from 87.2 moles sodium? EX. Vol. Part. Part. Given the balanced equation… Mass How many moles sodium are required to produce 0.736 moles sodium oxide? 7