Separate Mixtures 2
advertisement

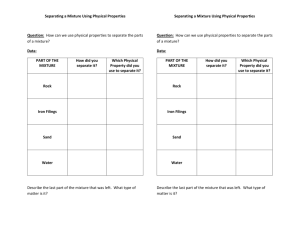
Name Class Date Lab: Separate Mixtures Background Information: A mixture is made up of two or more compounds or elements mixed together but not chemically combined. A mixture can be separated by a variety of methods such as evaporation, distillation, filtration and differences in solubility. This lab is designed for you to separate a mixture of an element and a compound using solubility and filtration. Objective: To separate a mixture of copper sulfate and sulfur. Materials: copper sulfate, sulfur, filter paper, two beakers, stirring rod, funnel, watch glass, balance. Procedure: Day 1: 1. Weigh out 2 grams of copper sulfate and 2 grams of sulfur. Record the physical properties (color, texture, state) of both substances in your data table. 2. Put the mixture of copper sulfate and sulfur into a beaker. Record the color of the mixture in your data table. 3. Add 100 mL. of water to your mixture. Stir your mixture for one minute. 4. Filter the mixture through a funnel fitted with filter paper. Catch the filtrate (liquid that goes through the filter paper) in a clean beaker. 5. After the mixture has been filtered, remove the filter paper and place it in an appropriate place to dry overnight. 6. Pour 1 mL. of the filtrate into a watch glass. Put the watch glass in an appropriate place to sit overnight. 7. Discard the remaining filtrate in an appropriate container. 8. Wash and dry your glassware. Day 2: 1. Observe the substance on the filter paper. Record its physical properties in your data table. 2. Observe the substance on the watch glass. Record its physical properties in the data table. 3. Discard the filter paper. 4. Wash and dry your watch glass. Data: Day 1 Copper sulfate Sulfur Mixture Day 2 Solid on filter paper Solid on watch glass Physical Properties (color, texture, state, etc.) Questions: 1. What was the name of the solid on the filter paper? 2. What was the name of the solid on the watch glass? 3. What property of the copper sulfate allowed you to separate the mixture easily? 4. When you mixed the copper sulfate and the sulfur with water, did they undergo a physical change or a chemical change? Explain. 5. Does a mixture have the same composition throughout? 6. How could you separate the following mixtures: a. Sulfur and iron filings: b. Salt and pepper: c. Gravel and sand: d. Sand and baking soda: e. Salt and water: f. Sugar and sulfur: g. Copper sulfate and sand: h. Copper sulfate and water: i. Alcohol and water: 7. Give an example of a mixture made up of two elements 8. Give an example of a mixture made up of an element and a compound 9. Give an example of a mixture made up of two different compounds 10. Could a compound be made up of two elements mixed together physically? Summary: Write a three paragraph conclusion summary using our standard format (paragraph one is what you were doing and why; paragraph two is what you found out; and paragraph three is how what you found out relates to your life.)