Elements Surround Us
advertisement

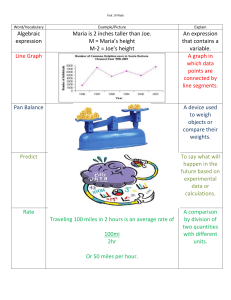
Elements Surround Us In addition to making up all living things, elements exist all around us in the atmosphere and the ocean. Most elements exist in molecular or ionic form. The elements most readily available are those occurring in the atmosphere. Table One lists the major components of clean, dry air. Table One: Composition of Clean, Dry Air Substance Nitrogen Oxygen Argon Carbon dioxide Neon Helium * parts per million Amount ppm* 781 000 208 000 9 300 300 20 5 Percentage 78.1 20.8 0.93 0.03 0.002 0.0005 These gases can be isolated by liquefying pure air. If air is cooled properly it will form a liquid solution. Once a liquid forms, each gas can be extracted or removed by distilling the liquid air. All the neon required for neon signs is obtained this way, along with large amounts of pure oxygen. The next most widespread source of the elements is the ocean. Table Two gives an analysis of seawater. Most elements in the ocean are found as electrically charged particles or ions. The most common are sodium and chloride ions, which give seawater its characteristics saltiness. Table Two: Typical Composition of Seawater Element Chlorine Sodium Magnesium Sulfur Calcium Potassium Carbon Bromine Amount ppb* 10 000 000 8 000 000 1 000 000 700 000 300 000 200 000 50 000 10 000 * parts per billion Element Boron Arsenic Copper Zinc Manganese Scandium Titanium Molybdenum Amount ppb* 7 000 10 10 10 5 2 1 1 As you become more comfortable with the language and symbols of chemistry, you will become more aware of the composition of substances that exist everywhere around you. Table Three lists the important elements that people require for bodily functions. If you were to add up the total cost of all the elements needed to make up a person of average mass (70 kg), it would come to approximately $154.00 (2013 data). Table Three: The Biological Role of Some Common Elements Element Fluorine Magnesium Phosphorus Calcium Cobalt Iodine Iron Role Required in small amounts for bony structures such as teeth Required for nerve and muscle action Required for biological energy A major requirement of bone Part of Vitamin B12 An essential part of the thyroid hormone Essential component of blood Symbols and Names Chemical symbols are used to convey ideas quickly and concisely. These shorthand notations are merely a convenience, and contain no mysterious concepts that cannot be expressed in words. It is interesting to read how the elements acquired their names. Believe it or not, scientists have never named an element they discovered after themselves. The names given to elements can Honour famous scientists. Curium was named after Marie Curie, the scientist responsible for discovering radioactivity. Indicate the country of discovery. Americium was discovered in America. Describe the element's properties. Krypton is Greek for hidden one because it is a very rare element. As more and more elements were being discovered, the need became evident for an organized system for discussing these elements among scientists in different countries. Scientists all over the world began to record their experimental results in journals so that these results could be read by all other interested scientists. It was important that all of the journals used common symbols to represent the elements. Some of the many different early symbols for the elements are shown in Figure One. Figure One: Early Symbols for Selected Elements Antimony Bismuth Copper Iron Gold Lead Mercury Silver Tin Zinc A Swedish chemist by the name of Jöns Berzelius recognized the need for a simple system to represent all the elements. In 1814 he devised a system that is now used by scientists all over the world. Berzelius took the first letter of each element and capitalized it: H represents hydrogen, U represents uranium, and C represents carbon. However, Berzelius encountered two problems. First, there were only 100 elements to name and only 26 letters in the alphabet. Secondly, the names of some elements began with the same letter. This problem was solved for elements beginning with the same letter by capitalizing the first letter and following it by a second, lower-case letter. As a result, most elements have been given two-letter symbols. Some examples of these are Be, beryllium; Ba, barium; and Bk, berkelium. Some elements have symbols other than the first letter in their name. The clue to these symbols is found in Latin, the scientific language. Listed in Table Four are eleven elements whose symbols are derived from Latin (L) and German (G) names. Table Four: Symbols and Elements with Foreign Derivatives Element Symbol Foreign Name Antimony Copper Gold Iron Lead Mercury Potassium Silver Sodium Tin Tungsten Sb Cu Au Fe Pb Hg K Ag Na Sn W Stibium (L) Cuprum (L) Aurum (L) Ferrum (L) Plumbum (L) Hydragyrum (L) Kalium (L) Argentum (L) Natrium (L) Stannum (L) Wolfram (G) Questions 1. What elemental gases are found in air? 2. Why is seawater salty? 3. Elements that living things require to survive are often called minerals. Describe the function in the body of two element minerals. 4. Why are chemical symbols used? 5. A. Describe the short-form system of element symbols developed by Jöns Berzelius. B. What advantage(s) does this system have over the short-form system used by earlier chemists? C. How many elements begin with the letter C? … the letter T? D. What letter is not found on the periodic table? E. Why don't the symbols of elements like lead (Pb) and tin (Sn) follow the system developed by Berzelius? 6. The names of the elements have many origins. A. Other than curium, state two elements named after other scientists. B. Other than americium, state two elements named after places.