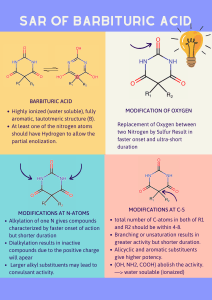
Outline of Material Covered
advertisement

ORGANIC CHEMISTRY II EXAM I MATERIAL REVIEW Spring Semester Week 4, 2013 I. Chapter 15: Radicals Free Radical Halogenation of Alkanes: Initiation-Propagation-Termination steps General Features of Free Radicals (more and less stable radicals) Selectivity in free radical halogenation Bromination (more selective) versus Chlorination (less selective) Hydrohalogenation of alkenes in the presence of peroxides (free radical mechanism) –“anti-markovnikov addition” Allylic Halogenation Reagents used (halogen free radicals; NBS) Product mixtures in allylic halogenation (whenever two different resonance structures can be drawn for an allylic radical) II. Chapter 16: Conjugation, Resonance, and Dienes Conjugation: minimum structures 1,3-dienes; allylic carbocations (remember allylic radicals too) Review of Resonance structures Classification of Dienes Electrophilic additions reactions of conjugated dienes (hydrohalogenation) 1,2- versus 1,4-addition Kinetic versus Thermodynamic factors Diels-Alder Reactions: Dienophile reactivity Stereospecificity Formation of bicyclic products (endo products are more favorable) Alkyne dienophiles III. Chapter 17: Benzene and Aromatic Compound Structural features of aromatic compounds Why is benzene not 1,3,5-cyclohexatriene? Criteria for aromaticity… Hückel’s Rule (4n + 2) Examples of aromatic compounds Annulenes Fused aromatic rings Aromatic Heterocycles: pyridine versus pyrrole Aromatic ions: Reactions which involve aromatic transition states (including those which are ionic –comparison of cyclopentadiene and cycloheptatriene derivatives) IV: Chapter 18: Electrophilic Aromatic Substitution (EAS) General mechanism (two step: addition followed by elimination) Reaction Profile (rate determining step is the formation of the carbocationic -intermediate) Common EAS Reactions: Nitration; Sulfonation; Halogenation; Friedel-Craft’s Alkylation; Friedel-Craft’s Acylation; Reactions with Carbocations Rearrangements in Friedel-Craft’s Alkylations EAS Reactions of mono-substituted benzenes (to give disubstituted benzenes) Effects of substituents towards rate of reaction: Activating versus Deactivating substituents Effects of substituents in the selectivity of the reaction (orientation of substitution: ortho-para or meta directing substituents) Determining the electronic effects of substituents: electron releasing versus electron withdrawing substituents Inductive (through -bonds) and resonance (through -bonds) effects Synthesis of disubstituted benzenes from benzene (which substituent to add first) Side Chain Reactions: Benzylic bromination (with NBS) Functionalizing the benzylic bromide further (to prepare alkenes, vic-dihalides, epoxides, alkynes, etc) Oxidation of alkyl benzenes (into benzoic acid) Reduction of aryl ketones to alkyl benzenes (Clemensen Reduction): a possible route to make alkyl benzenes after acylation (thus avoiding carbocation rearrangements) Multistep Syntheses Page 2 Page 3