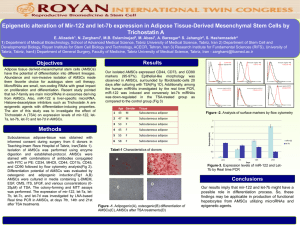
Study Year Tissue Comparison Subjects Pathway altered 3 Aligier
advertisement

# Study Year 3 Aligier 5 Baranova 7 Bouchard 9 Bujalska Tissue Comparison Subjects Pathway altered 2012 Subcutaneous adipose tissue collected by needle biopsy Day 0, day 14 and day 56 of overfeeding 13 healthy males (18 to 55yo) 2005 Omental adipose tissue obtained during bariatric surgery; the control samples from non-obese laparoscopic donor ongoing nephrectomy 2010 subcutaneous adipose tissue collected by needle biopsy Obese vs. nonobese 50 obese (9 type 2 diabetic), 9 non-obese Day 14: ↑ lipid metabolism, ↑ fatty acid, ↑ lipid biosynthesis; Day 56: ↑ lipid metabolism, ↑ cell adhesion, ↑ extracellular matrix, ↑ collagens, ↑ blood vessel development and morphogenesis, ↑ members of renin angiotensin family; ↓ Wnt/b-catenin signaling ↑ glycolisis enzymes; ↑ protein biosynthesis; ↑ cell cycle; ↓ oxysterol biosynthesis, ↓ angiogenesis highresponders vs. low-responders to weight reduction 2006 Preadipocytes from subcutaneous and omental adipose tissue preadipocytes were treated for 24 h with 100 nM cortisol (F) obese and overweight women, 7 low responders to weight loss and 7 high responder to weight loss five females (mean body mass index 29.4 kg/m2) undergoing hysterectomy expression in highresponders: ↑ cerebellar long-term depression pathway; ↓ protein transport, ↓ blood vessels development, ↓ angiogenesis ↑ regulation of adipocyte differentiation 10 Mutch 2009 Abdominal subcutaneous fat obtained by needle aspiration, surgical abdominal subcutaneous fat biopsies obese-needle vs. obese surgery; leanneedle vs. leansurgery 9 obese-needle, 9 obese surgery, 9 leanneedle, 10 leansurgery 12 Jernás 2006 Subcutaneous adipose tissue surgical biopsies and needle 12 lean subjects 20 Franck 2011 Adipose tissue biopsies from the abdominal subcutaneous depot and the major omentum large adipocytes (mean 100.1 ± 3.94 µm) vs. small adipocytes (mean 57.6 ± 3.54 µm) before (week 0), during (weeks 8 and 16) and after (week 18) treatment with a very low calorie diet 24 obese and 6 healthy Surgery and Needle: ↑ cytokine-cytokine receptor interaction,↑ FceRI signaling pathway, ↑ metabolism of lipids, carbohydrates and amino acids; ObeseNeedle vs. Lean-Needle: ↑ natural killer cell– mediated cytotoxicity,↑ antigen processing and presentation, ↓ cytokine-cytokine receptor interaction, ↓ complement and coagulation cascades, ↑ extracellular component; surgical biopsies: ↑ inflammatory ↑ cytoskeleton, ↑ cellular interaction pathways, ↓ metabolism of lipid, carbohydrate and amino acid, ↓ oxidative phosphorylation pathway. ↑ immune-related, ↑ structure regulated by caloric intake: ↑ lipid and fatty acid metabolism; ↓ protein synthesis, ↓ metabolism, ↓ lipogenesis, ↓ control of protein synthesis, ↓ β-oxidation, ↓ insulin resistance 25 Darimont 2008 mesothelial cells and in cell fractions from omental adipose tissue mesothelial cells vs. stroma vascular fraction vs. adipocytes 29 Lee 2005 Subcutaneous adipose tissue collected by needle biopsy Obese vs. nonobese OAT and SAT were collected in 11 obese Caucasian female patients undergoing gastroplastic surgery; for microarray 5 OAT 20 non-obese and 19 obese 30 Nair Obese vs. nonobese 14 non-obese and 14 obese 33 Hindle 2005 Preadipocytes from subcutaneous abdominal fat needle biopsies 2010 Omental adipose tissue samples obtained during bariatric surgery Diabetic obese vs. non-diabetic obese ↑ genes associated with cardiovascular, ↑ lipid metabolism, ↑ cellular development of the hematologic system development 36 Marrades Obese vs. lean 37 Marrades 2010 Abdominal subcutaneous adipose tissue biopsies collected by liposuction 2011 Abdominal subcutaneous adipose tissue biopsies collected by 20 morbidly obese patients undergoing bariatric surgery; 10 diabetic and 10 non-diabetic 9 lean and 9 obese, both high fat consumers 9 lean and 9 obese, both high fat consumers ↓ fatty acid oxidation, ↓ tricarboxylic acid (TCA) cycle, ↓ electron transport chain, ↓ fatty Obese vs. lean ↑ inflammatory-related genes ↑ inflammation/immune system, ↑ signaling transcription, ↑ cell cycle control/cell proliferation, ↑ cell adhesion, ↑ structural protein/cytoskeleton organization, ↑ apoptosis, ↑ proteolysis/peptidolysis, ↑ cell growth and maintenance, ↑ blood coagulation, ↓ energy energy pathway/electron transport ↑ inflammation ↓ basal and hormone stimulated lipolysis,↓ lipoprotein and cholesterol metabolism liposuction acid biosynthesis, ↓ glucose metabolism 45 MacLaren 2008 Subcutaneous adipose tissue was collected from the abdominal wall; and omental adipose tissue was collected from the greater omentum Subcutaneous IRO, n = 6, ISO + and omental NO = 10 adipose tissue paired from non-obese (NO), insulinsensitive obese (ISO) vs. insulinresistant obese (IRO) subjects 50 Wang 2009 Abdominal subcutaneous adipose tissue biopsies collected by needle Treated vs. Placebo 53 Yang 2004 Isolated adipocytes from subcutaneous adipose tissue Male individuals either insulinresistant and had at least two firstdegree relatives with type 2 diabetes or insulinsensitive and had no known family history of diabetes (controls) 72 obese men and women without family history of diabetes; 52 were treated with ephedra and caffeine (E+C) and 20 with placebo for 8 weeks 12 insulin resistant and 10 controls Omental: ↑ proteins involved in extracellular signaling, ↑ intracellular signaling, ↑ structural proteins, ↑ cell growth, ↑ differentiation, ↑ apoptosis, ↑ proteolysis, ↑ glucose transport, ↑ protein synthesis, ↑ proliferation, ↑ differentiation, ↓ intermediary metabolism and energy metabolism. ↑ purine metabolism, ↑ anion transport, ↑ cell adhesion, ↑ signaling pathway, ↑ cell sorting, ↑ protein targeting ↑ cell cycle-dependent transcriptional regulation, ↑ cytoskeletal reorganization, ↑ extracellular matrix remodeling, ↑ signal transduction, ↓ Wnt signaling genes, ↓ adipogenic transcription factors 55 Zhang 2007 Abdominal subcutaneous and omental adipose tissue 2010 Visceral adipose tissue 56 Wang 57 Walewski 2010 Omental specimen obtained during bariatric surgery 59 Urs 2004 Subcutaneous abdominal fat 60 Lee 2011 Culture of abdominal subcutaneous and omental adipose tissues 63 Disk 2009 subcutaneous adipose tissue obtained by needle biopsy 64 Geiger 2011 SGBS adipocytes 69 GómezAmbrosi 2003 Omental adipose tissue biopsies were obtained during laparoscopic surgery Omental vs. subcutaneous adipose tissue T2DM vs. control 10 massively obese men ↑ cell death Two male ↓ mitochondrial autopsy donors, OXPHOS one with type 2 diabetes mellitus and the other without type 2 diabetes mellitus Obese vs. non- 10 obese and 7 ↓ lipolysis, ↓ βobese non-obese oxidation, ↓ metabolism of fatty acids Adipocytes vs. 6 non-obese ↑ lipid metabolism pre-adipocytes women undergoing elective surgeries or liposuctions. Adipose tissue 4 severely ↓ treated with obese subjects immune/inflammatory, insulin and ↓ pro-apoptotic dexamethasone pathways, ↑ acutevs. treated with phase response, ↑ insulin stress/defense response SFA-rich diet vs. 20 abdominally ↑ inflammation MUFA-rich diet overweight subjects; 10 per group Hypoxic vs. _ ↑ glycolysis, ↑ normoxic cells response to hypoxia, ↑ regulation of cellular component movement, ↑ response to nutrient levels, ↑ regulation of cell migration, ↑ transcription regulator activity Obese vs. lean 6 male patients ↓ growth factors pooled for the obese and lean groups 70 Mustelin 2008 Subcutaneous adipose tissue biopsies collected by needle biopsy Obese vs. nonobese co-twins 71 Wolfs 2010 Omental adipose tissue biopsies were obtained during surgery Visceral vs. subcutaneous adipose tissue 74 Szalowska 2011 Omental adipose collected during gynecological surgery was cultured in 100 µg/ml of LPS during 24h. LPS vs. control 18 pairs with a reported BMI difference of at least 4 kg/m2, co-twin was non-obese whereas the other was obese and 10 concordant twins 20 T2D + NASH, 25 T2D, 41 NASH, 29 no T2D + no NASH 7 healthy caucasian females ↓ mitochondrial oxidative phosphorylation ↑ signal transduction, ↑ cell adhesion, ↑ cell communication, ↑ developmental processes, ↑ lipid, fatty acid, and steroid metabolism, ↑ cell structure and motility, ↑ developmental processes, ↑ cell adhesion,↑ immunity and defense, ↑ signal transduction, ↑ cell adhesion-mediated signaling ↑ chemokines, ↑ growth and differentiation of hematopoietic precursors, ↑ antiapoptosis, ↑ modulation of immune response, ↑ extracellular matrix remodeling, ↓ lysosomal/endosomal system activity, ↓ basement membrane components, ↓ extracellular matrix components, ↓ cell adhesion and migration, ↓ deoxyribonucleases activity, ↓ detoxification 75 Tienen 2011 Preadipocyte cell cultures were prepared from subcutaneous fat biopsies collected by liposuction 77 Dankel 2010 Subcutaneous adipose tissue biopsies collected during surgery 80 Kolehmainen 2008 Subcutaneous adipose tissue collected by needle biopsy Shah 2009 Serial subcutaneous adipose samples were collected by needle aspiration, 30 min before and 4, 12, and 24 h after LPS 82 83 Kursawe 2010 Subcutaneous adipose tissue collected by needle biopsy T2DM vs. control seven T2DM obese patients and nine control obese subjects 16 patients (12 women) peroperatively and one year after surgery, and from 13 lean healthy control subjects who underwent inguinal hernia repair (6 women). Weight reduction vs. control Human endotoxixemia (LPS 3ng/kg) 30 min before and 4, 12, and 24 h after LPS; validation group: 0.6 ng/kg of LPS 0, 4, 12, 24h 9 weight reduction >5% and 10 controls 14subjects for human endotoxixemia (LPS 3ng/kg), 30 min before and 4, 12, and 24 h after LPS; 7 subjects for validation: 0.6 ng/kg 0, 4, 12, 24h High ( >0.11) vs. Thirty-eight Low (< 0.11) obese VAT/(VAT + adolescents; 16 SAT) ratio low and 18 high for microarray ↓ adipogenesis, ↓ extracellular matrix remodeling, ↓ actin cytoskeleton, integrin signaling genes, ↓ insulin signaling, ↓ lipid metabolism, ↑ inflammation, ↑ apoptosis Immunity and defense and Signal transduction were strongly overrepresented among down-regulated genes post-surgery; overrepresentation postoperatively of the Immunity and defense sub-categories B-cell, Tcell, and natural killer cell mediated immunity an ↓ extracellular matrix, ↓ cell death ↑ cell adhesion, ↑ chemokine and cytokine, ↑ acutephase response, ↑ Tcell activation, ↑ metal binding, ↑ lipid metabolism ↓ lipogenesis, ↓ adipogenesis markers 85 Klimcakova 2011 Paired subcutaneous and visceral adipose tissue from subjects undergoing abdominal surgery lean, overweight, obese, and obese with metabolic syndrome 8 lean, 8 overweight, 8 obese and 8 obese with metabolic syndrome 87 MárquezQuiñones 2010 Subcutaneous adipose tissue collected by needle biopsy 22 successful weight lost and 22 unsuccessful 88 Capel 2009 Subcutaneous adipose tissue collected by needle biopsy 90 Clément 2004 Subcutaneous adipose tissue collected by needle biopsy Successful weight lost compared to unsuccessful weight lost; between diet branches 1) an energy restriction phase, i.e., before versus after the VLCD, 2) a weight stabilization phase, i.e., after the VLCD versus after weight maintenance, and 3) the entire dietary intervention Obese subjects before and after 2 or 28 day VLCD 22 premenopausal women MS vs. LE: ↓ branchedchain amino acid, ↓ fatty acid, ↓ carbohydrate, ↓ mitochondrial energy metabolism, ↑ immune, ↑ angiogenesis, ↑ apoptosis; subcutaneous vs. omental: ↑ lipid, ↑ carbohydrate metabolism; omental vs. subcutaneous: ↑ immune response, ↑ cell death, ↑ angiogenesis ↑ OXPHOS, ↑ TGF-β signaling, ↑ cell proliferation During energy restriction: ↓ lipid, ↓ carbohydrate, ↓ mitochondrial energy pathways; during weight stabilization: ↑ lipid, ↑ carbohydrate metabolism, ↓ immunity and defense was decreased; Overall dietary intervention: ↓ immunity, ↓ tissue remodeling 29 obese ↓ pro-inflammatory caucasian factors, ↑ antiwomen, inflammatory molecules premenopausal, subjects 94 RodríguezAcebes 126 Cancello 2010 Subcutaneous adipose tissue collected from women undergoing bariatric surgery Obese vs. control 15 morbidly obese and 10 non-obese control patients 2005 Subcutaneous adipose tissue collected during adjustable gastric roux-em-Y bypass surgery and monocytes differentiated in macrophages Adipocytes from lean and morbidly obese subjects before and 3 months after bypass surgery; for macrophages: non-obese 7 lean and 10vmorbidly obese subjects before and 3 months after bypass surgery; monocytes from 15 nonobese were cultured and differentiated in macrophages ↑ transcription factors for early stages of adipocyte differentiation, ↓ transcription factors for late stages of adipocyte differentiation adipocytes: ↑ metabolism/ energy, ↑ lipid metabolism, ↑ glucose metabolism, ↑ secretory pathway; macrophages: defense response, ↑ chemotaxis, ↑ humoral immune response, ↑ antigen processing, ↑ inflammatory response,↑ regulation of cell proliferation, ↑ regulation of transcription, ↑ protein biosynthesis, ↑ extracellular matrix organization and biogenesis; after weight loss: ↑ complement related factors, ↑ major histocompatibility complex, ↓ defense and immune response, ↓ chemotactism, ↓ positive regulation of cell proliferation, ↓ cell-cell signaling