Option D HL past paper questions 2011
advertisement
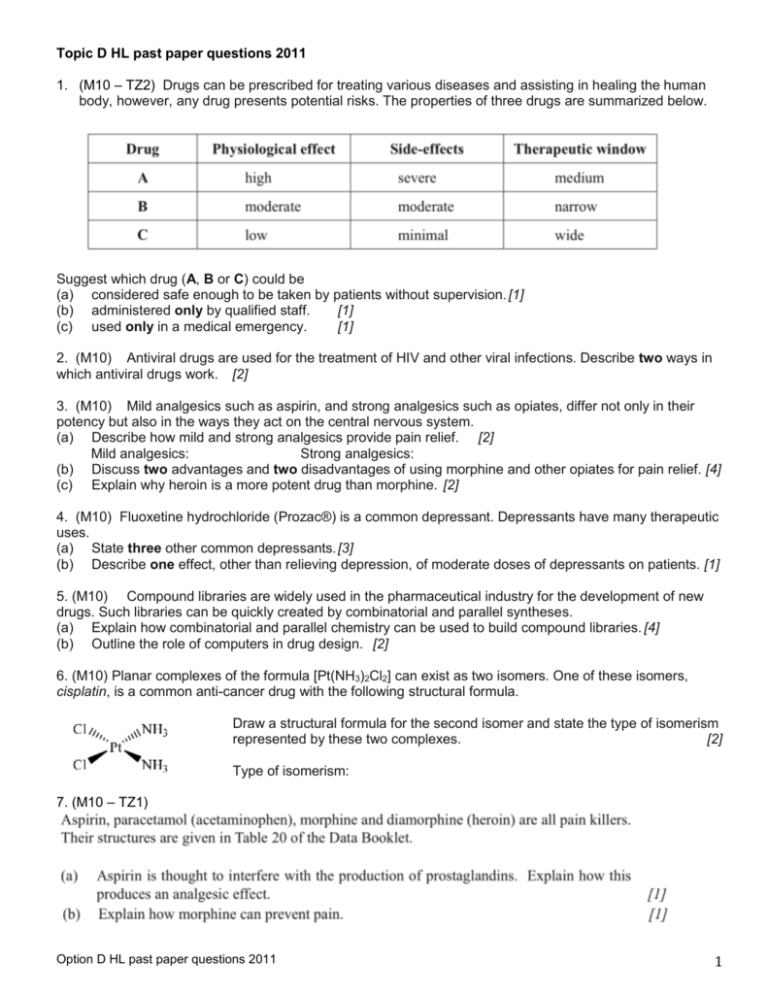
Topic D HL past paper questions 2011 1. (M10 – TZ2) Drugs can be prescribed for treating various diseases and assisting in healing the human body, however, any drug presents potential risks. The properties of three drugs are summarized below. Suggest which drug (A, B or C) could be (a) considered safe enough to be taken by patients without supervision. [1] (b) administered only by qualified staff. [1] (c) used only in a medical emergency. [1] 2. (M10) Antiviral drugs are used for the treatment of HIV and other viral infections. Describe two ways in which antiviral drugs work. [2] 3. (M10) Mild analgesics such as aspirin, and strong analgesics such as opiates, differ not only in their potency but also in the ways they act on the central nervous system. (a) Describe how mild and strong analgesics provide pain relief. [2] Mild analgesics: Strong analgesics: (b) Discuss two advantages and two disadvantages of using morphine and other opiates for pain relief. [4] (c) Explain why heroin is a more potent drug than morphine. [2] 4. (M10) Fluoxetine hydrochloride (Prozac®) is a common depressant. Depressants have many therapeutic uses. (a) State three other common depressants. [3] (b) Describe one effect, other than relieving depression, of moderate doses of depressants on patients. [1] 5. (M10) Compound libraries are widely used in the pharmaceutical industry for the development of new drugs. Such libraries can be quickly created by combinatorial and parallel syntheses. (a) Explain how combinatorial and parallel chemistry can be used to build compound libraries. [4] (b) Outline the role of computers in drug design. [2] 6. (M10) Planar complexes of the formula [Pt(NH3)2Cl2] can exist as two isomers. One of these isomers, cisplatin, is a common anti-cancer drug with the following structural formula. Draw a structural formula for the second isomer and state the type of isomerism represented by these two complexes. [2] Type of isomerism: 7. (M10 – TZ1) Option D HL past paper questions 2011 1 8. (M10) 9. (M10) Option D HL past paper questions 2011 2 10. (N09) The discovery of penicillin was one of the most significant scientific discoveries of the last century. (a) Describe the mode of action of penicillins in treating infectious diseases. [2] (b) Ampicillin is a semi-synthetic penicillin used to treat lung infections. The structure of the antibiotic is shown below. (i) Explain why it is important to continue to develop semi-synthetic penicillins. [2] (ii)Describe how computers are used in drug design. [2] (c) State the type of hybridization of each of the carbon atoms (I, II, and III) in the β-lactam ring of ampicillin by completing the table below, and explain why the amide group is highly reactive. [2] 11. (N09) (a) Explain the meaning of the terms: (i) side-effect [1] (ii) therapeutic window [1] (ii) placebo effect. [1] (b) The effectiveness of a drug depends on the method of administration. (i) One method of injecting drugs into the body results in the drug having a very rapid effect. State the method and explain its rapid action. [2] (ii) List the two other methods which can be used to inject drugs into the body. [1] (iii) Identify the method of administration used to treat respiratory diseases such as asthma. [1] 12. (N09) Amphetamine and methamphetamine are widely abused drugs. (a) State one short-term effect of amphetamine on the human body. [1] (b) Explain why amphetamine is classified as a sympathomimetic drug and relate this to its structure. [2] (c) Regular use of amphetamine and methamphetamine can lead to tolerance. Explain why this is potentially dangerous. [2] (d) Amphetamine exists as optical isomers. Describe how chiral auxiliaries can be used to synthesize only the desired enantiomeric form of a drug from a non-chiral starting compound. [3] Option D HL past paper questions 2011 3 (e) The structures of morphine and heroin are shown in Table 20 of the Data Booklet. Explain the increased potency of heroin compared to morphine. [2] 13. (M09- TZ1) Analgesics are used to relieve pain in the body. Aspirin and paracetamol (acetaminophen) are both mild analgesics. (a) Discuss the advantages of using aspirin instead of paracetamol. [2] (b) Compare how mild and strong analgesics relieve pain in the body. [2] (c) The structures of the strong analgesics morphine and heroin (diamorphine) can be found in Table 20 of the Data Booklet. (i) Identify the amine functional group in the morphine molecule below by drawing a ring around it.[1] (ii) Classify the type of amine present in morphine. [1] .................................................................. (iii) State the name of the functional group found in heroin but not in morphine. [1] (iv) Explain the increased potency of heroin (diamorphine) compared to morphine. [2] 14. (M09) Medicines and drugs alter the physiological state of the body including consciousness and coordination. (a) State one other effect of medicines and drugs on the body. [1] (b) Explain the meaning of the following terms: (i) therapeutic window. [1] (ii) tolerance. [1] (c) In recent years there have been significant advances in drug design, mainly due to the techniques of combinatorial chemistry and parallel synthesis. Discuss the use of these techniques, with reference to: (i) the advantage of the techniques compared to previous methods. [1] (ii) the methods used. [3] (iii) the difference between combinatorial chemistry and parallel synthesis. [1] 15. (M09) Ethanol is a depressant that is widely consumed in many societies. When consumed excessively it has a major impact on families and society as a whole. Other depressants such as diazepam (Valium®) may be prescribed by a doctor. (a) Describe the effect on the individual of consuming depressants at moderate and high doses. [2] (b) One problem associated with ethanol consumption is an increased risk of traffic accidents. Police in many countries use a breathalyser to test drivers. The breathalyser contains potassium dichromate(VI). (i) Describe the colour change of potassium dichromate(VI) when it reacts with ethanol. [1] (ii) State with a reason whether chromium in potassium dichromate(VI) is oxidised or reduced by ethanol. [1] 16. (M09) The structures of mescaline and lysergic acid diethylamide (LSD) are in Table 20 of the Data Booklet. Describe two effects of taking mescaline on the human body and discuss the structural similarities between mescaline and LSD that lead to some of their similar properties. [4] 17. (M09-TZ2) Medicines and drugs can influence the functioning of the body. Discuss the term therapeutic window. Your answer should include its meaning, a quantitative description and an explanation of wide and narrow therapeutic windows. [4] Option D HL past paper questions 2011 4 18. (M09) Chemists can change structures of substances in order to produce chemicals with desired properties. (a) Aspirin is virtually insoluble in water. Use Table 20 in the Data Booklet to explain how aspirin can be made more water-soluble. Write an equation for the reaction. [2] (b) Prozac®, fluoxetine hydrochloride, a depressant, is water-soluble and is made by reacting fluoxetine with hydrochloric acid. Use Table 20 in the Data Booklet to deduce the structure of fluoxetine. [1] 19. (M09) Ethanol, a depressant, is sufficiently volatile to pass into the lungs from the bloodstream. The roadside breathalyser test uses acidified potassium dichromate(VI) which reacts with any ethanol present in the breath and converts it to ethanoic acid. (a) (i) State the oxidation and reduction half-equations that occur in the breathalyser when ethanol is present in the breath. [2] (ii) Describe the colour change that occurs to the acidified dichromate(VI) if ethanol is present in the breath. [1] (b) Police use the intoximeter, an infrared spectrophotometer to confirm a roadside breathalyser test. Explain how the amount of ethanol is determined from the infrared spectrum. [2] 20. (M09) Antibacterials are drugs that kill or inhibit the growth of microorganisms that cause infectious diseases. The general structure of penicillin, an antibacterial, is given below. (a) With reference to the structure above, state what the letter R represents and discuss how penicillins can be made more resistant to the penicillinase enzyme. [2] (b) Describe the composition and structure of the beta-lactam ring in penicillin and explain its importance. [5] (c) Describe and explain one effect of overprescription of antibacterials. [2] 21. (M09) Describe and explain difficulties associated with solving the AIDS problem. [4] 22. (N08) Both aspirin and heroin are analgesics. Their structures are given in Table 20 of the Data Booklet. (a) Explain why both aspirin and heroin can be described as esters. [1] (b) Describe the mode of action for each of the two analgesics, aspirin and heroin. [4] (c) State a serious side effect of aspirin. [1] (d) The taking of heroin can lead to tolerance. Describe what is meant by tolerance and explain why it is a particularly dangerous problem with heroin. [2] 23. (N08) Magnesium hydroxide, Mg(OH)2 , and sodium hydrogencarbonate, NaHCO3, are two common antacids. (a) (i) State an equation for each of these antacids to show how they neutralise excess hydrochloric acid in the stomach. [2] (ii) Explain which will be more effective as an antacid: 0.01 mol of magnesium hydroxide or 0.01 mol of sodium hydrogencarbonate. [1] (b) Antacids often contain alginates and anti-foaming agents. Explain their functions. [2] Option D HL past paper questions 2011 5 24. (N08) (a) Outline the major contributions made by Florey and Chain in the development of penicillin. [3] (b) The original penicillin was penicillin G. State two advantages of producing penicillins with altered sidechains compared to the original penicillin G. [2] 25. (N08) 5-fluorouracil is a drug that has been used for several years to treat bowel and colon cancer. The structure of 5-fluorouracil is shown below with two of its functional groups circled and labeled A and B. (a) Identify the functional groups A and B. [2] (b) State and explain whether 5-fluorouracil can exist as geometrical isomers. [1] More recently cisplatin has supplemented or replaced 5-fluorouracil to treat these types of cancer. The structure of cisplatin is given in Table 20 of the Data Booklet. (c) Draw the structure of transplatin. [1] (d) Suggest why transplatin, unlike cisplatin, is not effective as an anti-cancer drug. [1] (e) The chemical structures of the following drugs are given in Table 20 of the Data Booklet: paracetamol (acetaminophen), ibuprofen, amphetamine, caffeine, lidocaine and mescaline. Deduce which of these six drugs can exist as enantiomers. [2] 26. (M08-TZ1) Antacid tablets are used to neutralize some of the hydrochloric acid in the stomach. Two substances commonly used in the tablets are calcium carbonate and sodium hydrogencarbonate. (a) Write an equation to represent each of these neutralization reactions. [2] (b) Explain, with reference to the equations in part (a), why a tablet containing 0.01 mol of calcium carbonate is more effective than one containing 0.01 mol of sodium hydrogencarbonate. [1] (c) Explain why alginates are often included in antacid tablets. [1] (d) Dimethicone is often included in antacid tablets because of its anti-foaming action. (i) Explain, with reference to the equations in part (a), why dimethicone is included. [1] (ii) Identify another base used in antacid tablets for which dimethicone need not be included. [1] 27. (M08) Some medicines prescribed by doctors are classified as depressants. These depress the central nervous system. One depressant not prescribed by doctors but widely used in some societies is ethanol. (a) Describe two long-term effects of consuming large quantities of ethanol on the human body. [2] (b) One method used to check whether a car driver has consumed ethanol is the breathalyser. This makes use of an oxidation reaction of ethanol. For this reaction, identify: the reagent used, the colour change and a possible organic product. [3] 28. (M08) The term penicillins is used to describe a range of substances that are effective against bacteria, but not against viruses. The general structure of penicillins is shown in Table 20 of the Data Booklet. The first substance to include the term penicillin in its name was penicillin G (benzylpenicillin), in which R is C6H5CH2 (a) Deduce the number of carbon atoms in one molecule of penicillin G. [1] (b) Some bacteria are resistant to penicillin G. (i) Explain how these bacteria are able to resist the effect of penicillin G. [1] (ii) Describe how the structure of penicillin G was modified to overcome this problem. [1] Option D HL past paper questions 2011 6 (c) (d) Explain how penicillins are able to destroy bacteria. [1] A doctor prescribes a broad-spectrum antibiotic for a patient, then some days later prescribes a narrow-spectrum antibiotic. (i) Describe what the doctor does to allow the medication to be changed to the narrow-spectrum antibiotic. [1] (ii) State the main disadvantage of using a broad-spectrum antibiotic. [1] 29. (N10) Dyspepsia, commonly known as indigestion, is due to excess acid in the stomach and can be treated using antacids. (a) State the name of the acid found in the gastric juices of the stomach. [1] (b) Two examples of antacids are aluminium hydroxide and calcium carbonate. State the equations to show the action of each antacid. [2] (c) Antacid medicines often contain alginates and anti-foaming agents. (i) Explain briefly how alginates prevent heartburn. [2] (ii) Explain why anti-foaming agents are added and state one example. [2] 30. (N10) (a) Two examples of mild analgesics are aspirin and paracetamol (acetaminophen). Paracetamol is often used as an alternative to aspirin. State one advantage and one disadvantage of the use of paracetamol. [2] (b) Examples of strong analgesics are morphine, codeine and diamorphine (heroin). Their structures are shown in Table 20 of the Data Booklet. (i) Identify two functional groups present in all three of these analgesics. [2] (ii) Identify one functional group present in morphine, but not in diamorphine. [1] (iii) State the name of the type of chemical reaction which is used to convert morphine into diamorphine. [1] 31. (N10) Three factors which can influence the mechanism of the action of a drug include geometrical isomerism, polarity and ring strain. (a) For each of the following drugs, identify which one of these factors is involved. [3] Increased potency of diamorphine compared to morphine: Penicillin: Cisplatin: (b) Explain the action of penicillin with reference to your answer in part (a). [2] (c) O Thalidomide has the following three-dimensional structure. (i) Identify the chiral centre in the thalidomide structure (above) with an asterisk (*). Option D HL past paper questions 2011 [1] 7 (ii) Discuss the importance of chirality in the action of this drug by comparing the effects of each of the two enantiomeric forms. [2] 32. (N10) Two of the best known mind-altering drugs are lysergic acid diethylamide (LSD) and psilocybin. Using Table 20 of the Data Booklet, outline the essential structural similarities between the two drugs and describe the short-term effect of psilocybin. [4] Option D HL past paper questions 2011 8