Syllabus – General Chemistry I
advertisement
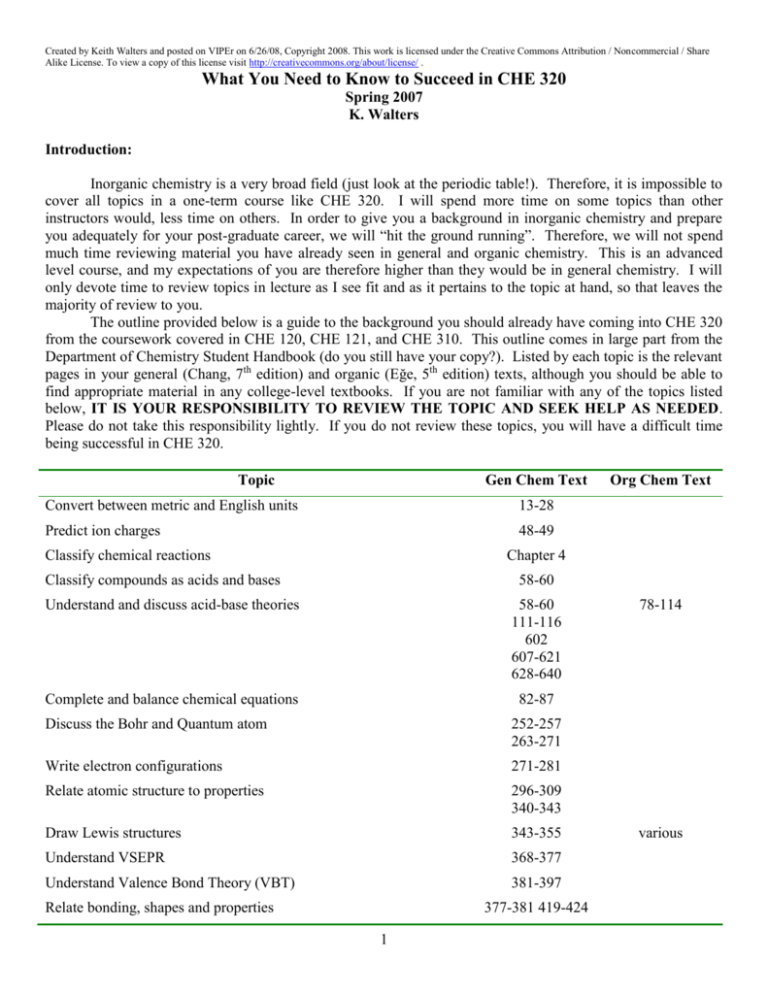
Created by Keith Walters and posted on VIPEr on 6/26/08, Copyright 2008. This work is licensed under the Creative Commons Attribution / Noncommercial / Share Alike License. To view a copy of this license visit http://creativecommons.org/about/license/ . What You Need to Know to Succeed in CHE 320 Spring 2007 K. Walters Introduction: Inorganic chemistry is a very broad field (just look at the periodic table!). Therefore, it is impossible to cover all topics in a one-term course like CHE 320. I will spend more time on some topics than other instructors would, less time on others. In order to give you a background in inorganic chemistry and prepare you adequately for your post-graduate career, we will “hit the ground running”. Therefore, we will not spend much time reviewing material you have already seen in general and organic chemistry. This is an advanced level course, and my expectations of you are therefore higher than they would be in general chemistry. I will only devote time to review topics in lecture as I see fit and as it pertains to the topic at hand, so that leaves the majority of review to you. The outline provided below is a guide to the background you should already have coming into CHE 320 from the coursework covered in CHE 120, CHE 121, and CHE 310. This outline comes in large part from the Department of Chemistry Student Handbook (do you still have your copy?). Listed by each topic is the relevant pages in your general (Chang, 7th edition) and organic (Eğe, 5th edition) texts, although you should be able to find appropriate material in any college-level textbooks. If you are not familiar with any of the topics listed below, IT IS YOUR RESPONSIBILITY TO REVIEW THE TOPIC AND SEEK HELP AS NEEDED. Please do not take this responsibility lightly. If you do not review these topics, you will have a difficult time being successful in CHE 320. Topic Gen Chem Text Convert between metric and English units 13-28 Predict ion charges 48-49 Classify chemical reactions Org Chem Text Chapter 4 Classify compounds as acids and bases 58-60 Understand and discuss acid-base theories 58-60 111-116 602 607-621 628-640 Complete and balance chemical equations 82-87 Discuss the Bohr and Quantum atom 252-257 263-271 Write electron configurations 271-281 Relate atomic structure to properties 296-309 340-343 Draw Lewis structures 343-355 Understand VSEPR 368-377 Understand Valence Bond Theory (VBT) 381-397 Relate bonding, shapes and properties 377-381 419-424 1 78-114 various Created by Keith Walters and posted on VIPEr on 6/26/08, Copyright 2008. This work is licensed under the Creative Commons Attribution / Noncommercial / Share Alike License. To view a copy of this license visit http://creativecommons.org/about/license/ . Describe hybridization and hybrid orbitals 385-394 Describe multiple bonds in terms of VBT 394-397 Describe resonance, write resonance forms 349-350 406-408 Relate state of matter to properties 418 424-428 438-443 Understand and discuss redox reactions 116-128 766-768 Understand kinetic theory of reactions 510-541 Understand principles of reaction equilibria Chapter 14 652-655 671-687 Discuss and calculate entropy, enthalpy, and free energy 735-755 Discuss electrochemical cells 769-771 Calculate cell potentials 771-784 Classify organic functional groups Chapter 24 various Classify organic reactions various Understand and apply reaction mechanisms 115-143 Describe the stereochemical structures of substances 191-232 Draw 3D structures of molecules Describe the stereochemistry of reactions 191-232 Name organic compounds various Name basic inorganic compounds 53-61 Where to Seek Help: If you need help on any of the topics listed below, do not panic! I am more than willing to help you on any of these topics, but due to our limited time I prefer to cover these topics outside of the normally scheduled lecture. Feel free to come by my office, make an appointment, send an email, or attend a review session. Dr. Niewahner is also available for help on these topics. What is crucial is that you take the first step to initiate help…I can’t read your mind! 2











