Organic Synthesis
advertisement

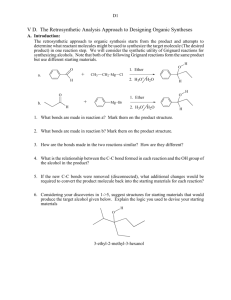
Organic Synthesis: Synthesis is the most creative thing we do in organic chemistry. Nomenclature is a straightforward application of rules, most spectroscopic interpretation, at this level, requires little in the way of judgment. Structure and properties of compounds are matters of simple observation. Synthesis, though, has some science and some art to it. There is rarely a ‘right’ answer. Most synthetic problems have more than one solution, and the trick is to judge which of these is likely to have the best chance of success. Even the most experienced chemists develop routes, which work well on paper but fail miserably in the lab. But, that having been said, there are some guidelines, which are helpful in designing a synthesis. What follows is an attempt to lay out those guidelines with a few examples. The best approach to designing a synthesis of a molecule when we have a choice of starting materials is to work our way back from the target to simpler compounds. This is called retrosynthesis. We try to imagine what might have been the last step in the synthesis. The starting material for this reaction becomes our new target. What was the last step in its preparation? We go backwards like this until we reach an acceptable starting material. Each of these backward steps is called a transform, and for each transform, there is a corresponding reaction. In the first part of the course, we don’t have very many reactions (and thus, transforms) available to us. Let’s assume that we have only gotten to the halogenation of alkanes, and the Corey-House reaction. We can therefore make alkyl halides, and couple them together to make larger alkanes. Let’s try to make some larger alkanes. Say we were asked to make hexane from butane. How do we even start? If you have been given a specific starting material and product, as in this case, try to identify the carbon atoms of the starting material in the product. * * * * * * * * We can see that we can have two arrangements. In the first one, two carbons (an ethyl group) have been attached to the end of the butane from which we started. In the other one, two one carbon groups (methyl groups) have been attached, one to each end. So we can have two approaches, one of which requires the formation of two C-C bonds, one which requires installing only one such bond. Let’s look at the one that requires the fewer bond-forming steps, on the grounds that we can probably do it in a smaller number of steps. How then can we make the target molecule? Look at what needs to be accomplished. We have to attach a two-carbon fragment to a four-carbon fragment. The only method available to us is the Corey-House reaction. Can we carry it out? This reaction requires the use of two alkyl halides, as shown below. CuCl Li 2 2 Cl Li 2 CuLi Cl Since we are required to start from butane, this doesn’t work. On the other hand, we know we can make alkyl halides from alkanes, and we’ve just shown how to make hexane from chlorobutane, so we can presumably get from butane to hexane, not directly, but via chlorobutane. In reality, most synthetic problems are like this. Intermediate compounds lie along the pathway between available starting materials and targets. Of course, this isn’t a synthesis you would be likely to undertake. First, hexane is cheap and readily available. Second, chlorination of alkanes isn’t completely selective. We need to consider the efficiency of each step along the way in order to decide if a synthesis is reasonable. Consider the preparation of 2methylpentane from alkanes of four carbons or less. We can imagine the source of the carbons as shown below * * * * * * (a) * (b) * * * * (c) Of the three options, the last involves forming 2 new C-C bonds and so is the least attractive. Of the other two, which to choose? If we are limited to Corey-house and free radical halogenation, as in the previous case, then we have the following two retrosyntheses, and their corresponding syntheses. Corey-House X halogenation + X Br 1. Li Br2 2. CuBr Cl2 Cl Cl + X Cl2 Cl + + X Cl 1. Li Cl2 Cl 2. CuBr Now, which route is the better? Here we run into the problem of selectivity. Halogenation of alkanes is a somewhat selective process. Hydrogens on more subsituted carbons are more easily replaced than those on less substituted carbons. Chlorination is less selective in this way than bromination. Thus, for the second route, chlorination of 2methylpropane yields both 2-chloro-2-methylpropane (which we don’t want) and chloro- 2-methylpropane (which we do want). The undesired product is produced in greater yield, so we have a problem with this route. Now look at the first route. We take advantage of bromine’s greater selectivity for the secondary position to make 2bromopropane. Chlorination gives us a mixture of products, the ratio of which can be manipulated a bit by varying the temperature. At room temperature, the ratio is about 6:4 in favour of the undesired product. At higher temperatures, we can get more of the desired chloropropane. So, given the choice between these two routes, we can most likely get the better yield of desired product using the first. We will find that, in general, branch points are good paces to make disconnections. This follows the common observation that disconnections that simplify more are often better than those which simplify less. OH O H C C C C OH O C H C C C Often, as in this case, you have options regarding the fate of the starting carbon atoms. Now, determine what has to happen to the starting material to get to the product in each case. In the first route above, we see that the carbonyl group has to be reduced to a hydroxyl, and then a methyl group has to be added to the same position. In the second route, the hydroxyl has to be reduced, and a methyl group has to be added to the other end of the chain. Which is preferred? This is where the art comes in. As a general policy, it is best not to try to carry out specific reactions on unfunctionalized positions. If you want a reaction to occur at a specific place, it is best if there is already a functional group at that location. Furthermore, only a limited set of reactions will lead to the desired outcome. So, at first glance, the best choice of the two is the first. Now what? For a relatively simple transformation like this one, it is a good idea to see if you can accomplish the synthesis in one step. In this case, is there a reaction, which does the following: 1. Form a carbon-carbon bond. 2. Form that bond to a carbonyl carbon. 3. Result in the conversion of that carbonyl group to a secondary hydroxyl group? At this point, a good grasp of synthetic reactions is important. You need to be able to look at that list of criteria and come up with a yes or no answer to the question “Can I do this in one step?” To help with this process, find reactions, which satisfy one criterion. Then eliminate those, which do not satisfy the next requirement, and finally the last. In this case, it’s probably a good idea to look at the rather limited number of C-C bond forming reactions that we know. These could be: 1. Corey-House: makes C-C bond, needs alkyl halide, makes alkane 2. Grignard reaction with a carbonyl: makes C-C bond, requires a carbonyl in the starting material, makes an alcohol. 3. Wittig reaction: makes C-C bond, requires a carbonyl, makes an alkene (which could be converted to alcohol later) 4. Grignard reaction with epoxide: makes C-C bond, requires epoxide, gives alcohol, adds 2 carbons to the chain. Of these options, only 2 and 3 look promising, and, of them, 2 is simpler. So one route to the desired product might be the following: O- MgBr+ OH H+, water O + CH3MgBr CH3 H The methyl group has been written out in full to make it obvious. The same result could be obtained using option 3, the Wittig reaction: CH3 OH O H + acid R3PCH2 water In practice, the Grignard reagent is more convenient, and the synthesis is accomplished all in one pot, the addition of acid and water occurring during the workup. The second route requires isolation of the alkene, probably by distillation (boiling point about 30o C). This last point illustrates that, once a viable set of reactions has been found, it is still necessary to consider the efficiency, convenience and cost of the process (at least in a real lab situation). Now try the following conversions, which can be accomplished by essentially one step processes (that is, without isolation of intermediates). CH3 O (a) O H (b) O (c) MgBr OH (d) O Most synthesis problems which we see in the lab are not quite so straight forward as the one above. Usually, we have a target compound, but no specific starting material. Instead, we try to come up with a synthesis from “readily available materials”. What do we mean by that? It may simply mean, “what’s around the lab”, but for our purposes, we’ll say that it means materials of about four or fewer carbons. All isomers of most kind of molecules in this size range are cheap and easy to find. This includes the alcohols, ethers, acids, alkyl halides, aldehydes, ketones, alkenes and alkynes. We usually also include benzene and its simple derivatives, such as toluene, phenol and benzoic acid. For example, imagine we had to prepare 3-hexanol from alcohols of four carbons or less. Here is a sample retrosynthesis, and the corresponding reaction sequence. Retrosynthesis: a + MgBr O OH b c OH Br d HO Reaction sequence: OH OH + PBr3 PCC b Mg d Br c O MgBr a 1. O 2.acid, water OH There are a number of things to point out here. First, notice that for each transform (a, b, c, d) there is a corresponding reaction. The last reaction matches with the first transform, the second last reaction with the second transform, and so forth. Second, details like reaction conditions are not usually written into transforms. In fact, many people don’t even put in the actual compounds. Rather, they just have skeletons indicating the electrophilic and nucleophilic sites, and try to infer reactions from there. Although that’s the current convention, for this document, we’ll use explicit compounds, at least for a while. How was that retrosynthesis done? Notice that the restriction on starting materials requires that at least one C-C bond be made. It could be more than one, but at least one is required. So, of all the reactions which are available to the organic chemist, we can be sure that we need one of the relatively small group of C-C bond forming reactions. Second, the product must contain an alcohol group. That may have survived from the starting material, or been produced along the way. One good tip is to try to find disconnections that simplify the molecule a lot, especailly disconnections at branch points. In this case, a disconnection at the carbon bearing the OH is a good idea. Now, what reactions form a C-C bond and result in an alcohol at that point? The reaction of a Grignard with an aldehyde will give a C-C bond and a secondary OH group. Other reactions might work, too. A Wittig reaction on the aldehyde would make the bond, but leave you with an alkene, which could be hydrated to the alcohol. This means an extra reaction step, and it isn’t clear which carbon will get the OH group. A Corey-House reaction will make the C-C bond, but leave you with an alkane. Look at each C-C bond forming reaction and consider the consequences. Having chosen the Grignard transform, we then have two new targets: the aldehyde and the Grignard. The aldehyde can be made from an alcohol. The Grignard reagent requires an alkyl halide, which then becomes a target. Fortunately, it is easy to make an alkyl halide from an alcohol, and there we are at a set of acceptable starting materials.