Supplementary material
advertisement

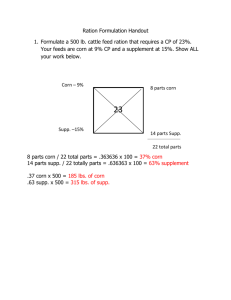
Supplementary material Methods Barley genotype analysis using Bgh. PCR markers for MLO, ROR1, and ROR2 have been described1,2 (and GenBank AY246906). Lines for MLO ror analysis were marker-selected from progeny of ror mutant (background BCIngrid mlo-5) Sultan5 (MLO ROR1 ROR2) crosses. Four sibling F4 families of each genotype were used in each comparison. Penetration frequencies were determined 48 hours after inoculation with the K1 Bgh isolate, as previously described1. Per family, 50 interaction sites were scored on each of 10 leaves per cell type. For the mlo ror1 ror2 analysis, ror1-4 and ror2-1 mutants were crossed, and resistance assays and the ROR2 marker were used to identify an F2 plant that was homozygous for the recessive ror1-4 allele at the ROR1 locus, and heterozygous at the ROR2 locus. Individuals from the next generation were used in penetration tests (50 sites scored for each of 8-20 leaves per genotype), after ROR2 marker genotype determination, or rated for fungal mycelium coverage, before ROR2 marker genotype determination. ROR2 syntenic map-based cloning. The ROR2 mapping population representing 1,522 meioses was constructed essentially as described2 using a cross between the Ethiopian landrace barley Grannenlose Zweizeiligge (mlo-11 ROR2) and the ror2-1 mutant (A44) derived from BCIngrid mlo-5. A set of 49 pre-mapped barley microsatellite markers3 (primers kindly provided by Mike Gale, John Innes Centre) were screened for close linkage to ROR2 using DNA pools made from homozygous ROR2 and ror2-1 segregants. ROR2 was mapped between the Triticeae markers wg644 and abg391 (Supplementary Fig. 2a) which were anchored by sequence searches to a contig of rice chromosome 3 BAC clones being sequenced by The Institute for Genomic Research (TIGR; http://www.tigr.org/). Potential rice gene sequences from the syntenic interval were used in BLASTN searches4 to identify EST sequences of close Triticeae homologues which were then used to generate PCR-based markers in barley as described2. The PCR protocol was modified by including 5% DMSO to assist amplification. Sequences of barley genes and details of PCR markers can be found in the GenBank entries indicated in Supplementary Fig. 2a. GFP-PEN1 fusion protein analysis. The binary GFP-PEN1 transformation construct contained the GFP ORF from pEGFP (Clontech) fused to the 5’ end of the PEN1 cDNA coding sequence, flanked by 1,224 bp of PEN1 5’ untranslated sequence and the NOS terminator. The ORF encoding the GFP-PEN1 fusion was confirmed by sequencing and for its ability to complement the penetration phenotype in the pen1-1 mutant after stable transformation (data not shown). Confocal images of leaves from transformants were collected using a Zeiss LSM Pascal laser-scanning microscope. Leaves were mounted in 0.85 M sodium chloride for 10 minutes to achieve plasmolysis. ROR2 antibody and ROR2 localization. The ROR2 antiserum was raised against the peptide 9WKRAGAGGDGDLESG23 in rabbit, affinity-purified (Eurogentec, Belgium), and used for protein blots at a dilution of 1:1,000. General barley protein techniques and other antisera were as described5. Figure S1 Bgh susceptibility of barley genotypes. a, ROR1 and ROR2 contribute to basal penetration resistance in the presence of wild-type MLO. Long and short adaxial epidermal cells with different levels of penetration resistance6 were assessed separately. b, Mutations in ROR1 and ROR2 have accumulative effects. left panel, Success of Bgh primary penetration attempts. *Different at P < 0.15. right panel, Visual ratings of fungal mycelium density on individual plants five days after inoculation. 8 = fully susceptible, 1 = fully resistant. Figure S2 Barley-rice syntenic map-based cloning of ROR2. a, Syntenic mapping. Lines connect barley genes (GenBank accessions indicated) on chromosome 5H to their orthologues on a BAC contig from rice chromosome 3. Marker names in bold appear on previously published cereal RFLP maps. Question marks denote markers mapping to unlinked locations in barley. Numbers of meioses screened and recombinants identified are shown for each interval. b, The ror2-1 EMS derived mutant allele contained both a deletion and a silent single base (GA) substitution. The deletion occurred at a 4 bp direct repeat (arrows), introduces a C (underlined) at the junction, and results in a 31 amino acid in-frame deletion. Nucleotide positions in the single ORF and in the protein are indicated. Note that although most EMS derived mutant alleles result from single nucleotide substitutions, several percent contain deletions or mutations at two sites7. Figure S3 ROR2 and PEN1 are located in the plasma membrane. a, Confocal images of transgenic Arabidopsis leaves expressing GFP-PEN1 fusion protein, before (upper panel) and after (lower panel) plasmolysis. Fluorescence was also visible in the plasma membrane extensions (Hechtian threads; he) connecting the plasma membrane (pm) to the cell wall (cw) of plasmolysed cells. Other structures: epidermal cells (ep), stomate (st), mesophyll cell (me). Bars = 20 m. b, Leaves of different barley genotypes were used to derive protein fractions by differential centrifugation and twophase partitioning to enrich for plasma membrane. The ROR2 antibody detects products of wild-type ROR2 and mutant ror2-1 alleles (arrows) around the respective expected molecular weights of 34.5 and 31.4 kDa. Duplicate blots were probed with antibodies for the plasma membrane -specific H+ATPase and CALRETICULIN (CALR.) located mainly in the lumen of the E.R. (control H+-ATPase and CALR. blots for MLO ROR2 and MLO ror2-1 genotypes not shown). An arrow identifies the CALR.-specific signal, distinguishing it from the higher molecular-weight non-specific signal which is also detected with this antibody8. Note that the mutant mlo-5 allele altered neither the abundance nor the localization of ROR2. Figure S4 Rat neuronal SNAP25 (Rn25), and homologues HvSNAP34 (Hv34) and HvSNAP28 (Hv28) from barley, and AtSNAP33 (At33) from Arabidopsis. A third barley SNAP25 homologue is not shown here due to incomplete sequence. Positions in the Qb and Qc -type SNARE domains of Rn25 that contribute to stabilizing ionic or hydrophobic interactions with other SNARE proteins9,10 are shown by a star and bullets, respectively. References. 1. Peterhänsel, C., Freialdenhoven, A., Kurth, J., Kolsch, R. & Schulze-Lefert, P. Interaction analyses of genes required for resistance responses to powdery mildew in barley reveal distinct pathways leading to leaf cell death. Plant Cell 9, 1397–1409 (1997). 2. Collins, N. C. et al. Sequence haplotypes revealed by sequence-tagged site fine mapping of the Ror1 gene in the centromeric region of barley chromosome 1H. Plant Physiol. 125, 1236–1247 (2001). 3. Ramsay, L. et al. A simple sequence repeat-based linkage map of barley. Genetics 156, 1997–2005 (2000). 4. Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990). 5. Devoto, A. et al. Topology, subcellular localization, and sequence diversity of the Mlo family in plants. J. Biol. Chem. 274, 34993–35004 (1999). 6. Koga, H., Bushnell, W. R. & Zeyen, R. J. Specificity of cell type and timing of events associated with papilla formation and the hypersensitive reaction in leaves of Hordeum vulgare attacked by Erysiphe graminis f. sp. hordei. Can. J. Bot. 68, 2344–2352 (1990). 7. Anderson, P. Mutagenesis. Methods in Cell Biology, Vol 48 48, 31–58 (1995). 8. Napier, R. M. et al. Purification, sequencing and functions of calreticulin from maize. J. Exp. Bot. 46, 1603–1613 (1995). 9. Fasshauer, D., Sutton, R. B., Brunger, A. T. & Jahn, R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Qand R-SNAREs. Proc. Natl Acad. Sci. USA 95, 15781–15786 (1998). 10. Sutton, R. B., Fasshauer, D., Jahn, R. & Brunger, A. T. Crystal structure of a SNARE complex involved in syntaptic exocytosis at 2.4Å resolution. Nature 395, 347–353 (1998).