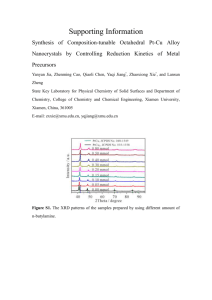
SUPPORTING INFORMATION Synthesis of ligands: Benzimidazol
advertisement

SUPPORTING INFORMATION Synthesis of ligands: Benzimidazol, (L1): o-phenylene diamine (6.83 g, 63.2 mmol) and formic acid (3.27 ml) were placed in a reaction tube and heated under reflux for 1 h. at 100 °C. After cooling to room temperature, the reaction mixture was diluted with distilled water (20 mL) and NaOH solution was added until the mixture was slightly basic. The precipitate was filtered and washed with cold water. The residue crystallized from hot water. Yield: 60%, 4.48g. m.p.: 171 °C. IR(cm-1): 3411, 3114, 3096, 3062, 3038, 3002, 2967, 2942, 2884, 2881, 2790, 2741, 2661, 2621, 2608, 2541, 1620, 1588, 1495, 1477, 1457, 1406, 1364, 1346, 1301, 1271, 1244, 1202, 1157, 1133, 1112, 1003, 958, 932, 887, 874, 847 ; 768, 744. UV(nm): 208, 244, 270. 1-Benzyl-1H-benzimidazol, (L2): Benzimidazole (0.5 g, 4.2 mmol) was dissolved in acetone. Then, KOH (0.24 g, 4.2 mmol) was added and refluxing acetone continued for 3 hours. After cooling to room temperature, benzyl chloride (0.6 ml, 4.3 mmol,) was added and refluxing acetone continued for 1 h. The volatiles were removed under vacuum. Residues were treated with 10 mL of distilled water and filtered with a cannula. The residue was crystallized from methanol/diethyl ether. Yield: 65%, 0.56g. m.p.: 115 °C. IR (cm-1): 3081, 3030, 3018, 2971, 2943, 2926, 1613, 1584, 1493, 1452, 1443, 1428, 1365, 1333, 1286, 1265, 1229, 1217, 1203, 1188, 1029, 974, 887, 776, 765, 739, 730, 694. UV(nm): 213, 251, 272. 1-(2,3,5,6-Tetramethylbenzyl)-1H-benzimidazol, (L3): L3 was synthesized according to L2 synthesis method using benzimidazole (0.5 g, 4.2 mmol), KOH (0.24 g, 4.2 mmol) and 2,3,5,6-tetramethylbenzyl chloride (0.78 g, 4.2 mmol). Yield: 75%, 0.83g. m.p.: 180 °C. IR (cm-1): 3066, 3002, 2968, 2941, 2917, 2862, 1612, 1586 , 1497, 1474, 1458, 1385, 1326, 1301, 1287, 1226, 1210, 1202, 1193, 1168, 1153, 1145, 1121 , 1085, 1034, 1009, 974, 962, 940, 927, 901, 889, 879, 846, 805, 763, 744, 739, 718, 686, 655. UV(nm): 211, 250, 273. Bzimpy, (L4): o-H3PO4 (20 mL) and P2O5 (10 g) were placed in a 500 mL were placed in a reaction tube and heated to 120 °C for 1 day. Then pyridine-2,6-dicarboxylic acid (3.376 g, 20.2 mmol) and o-phenylendiamine (4.84 g, 44.7 mmol) was added and heated to 190 °C for further 1 day. At the end of this time, the reaction mixture was cooled to room temperature. Then, the colored melt was slowly poured into 250-300 mL of vigorously stirred cold water. The bulk was collected by ordinary filter paper. The residue was washed three times with cold water and dried. The precipitate was treated with hot 10% aqueous Na2CO3 solution. The residue was filtered, washed with cold water and recrystallized from MeOH. Yield: 60%, 3.77 g. m.p.: >280 °C. IR (cm-1): 3141; 3055; 2971; 1622, 1600; 1592; 1572; 1456; 1434; 1409; 1384; 1374; 1317; 1278; 1229; 1216; 1157; 1148; 1113; 1013; 994; 962; 916; 901; 890; 879; 844; 818; 804; 764; 725; 686; 654. UV(nm): 219, 311. 2,6-Bis(1-benzyl-1H-benzo[d]imidazol-2-yl)pyridine, (L5): L4 (0.5 g, 1.14 mmol) and KOH (0.145 g, 3.0 mmol ) were dissolved in acetone. The mixture was stirred under reflux for 3 h. Then, benzyl chloride (0.26 ml, 2.28 mmol) was added and stirred under reflux for 6 h. At the end this time, the volatiles were removed under vacuum. Residues were treated with 10 mL of distilled water and filtered off. The residue was crystallized from methanol/diethyl ether. Yield: 72%, 0.380 g. m.p.: 240 °C IR(cm-1): 3203, 3064, 2967, 2924, 1613, 1590, 1571, 1437, 1409, 1381, 1331, 1316, 1278, 1261, 1252, 1231, 1169, 1114, 1099, 1083, 1009, 994, 902, 879, 870, 844, 824, 766, 736, 729, 689, 670, 663. UV(nm): 215, 316. 2,6-Bis(2,3,5,6-tetramethylbenzyl-1H-benzo[d]imidazol-2-yl)pyridine, (L6): L6 was synthesized according to the L5 synthesis method using L4 (0.5 g, 1.14 mmol), KOH (0,145 g, 3 mmol) and 2,3,5,6-tetramethylbenzyl chloride (0.420 g, 2.3 mmol). Yield: 76%, 0.734 g. m.p.: 263 °C IR(cm-1): 3067, 3010, 2971, 2939, 2916, 2864, 1614, 1588, 1570, 1484, 1475, 1438, 1408, 1378, 1330, 1289, 1262, 1250, 1217, 1170, 1151, 1121, 1099, 1083, 1041, 1018, 1008, 994, 964, 956, 924, 918, 903, 878, 836, 824, 758, 736, 699, 688, 669, 663. UV(nm): 225, 313. 1.00 0.95 0.95 0.90 0.90 0.85 0.85 0.80 0.80 0.75 0.75 0.70 0.70 0.65 0.65 0.60 0.60 0.55 Absorbance Absorbance 1.00 0.50 0.45 0.55 0.50 0.45 0.40 No 1 2 3 0.35 File Name MYI-L1.JSP MYI-L2.JSP MYI-L3.JSP 0.40 Color No 1 2 3 0.35 0.30 0.30 0.25 0.25 0.20 0.20 0.15 0.15 0.10 0.10 0.05 0.05 0 File Name MYI-K1.JSP MYI-K2.JSP MYI-K3.JSP Color 0 200 250 300 350 400 450 500 550 600 Wavelength (nm) 650 700 750 800 850 900 200 250 300 350 400 450 500 550 600 Wavelength (nm) 650 1.00 1.00 0.95 0.95 0.90 0.90 0.85 0.85 0.80 0.80 0.75 0.75 0.70 0.70 0.65 0.65 0.60 0.60 0.55 0.50 No 1 2 3 0.45 0.40 File Name MYI-L4.JSP MYI-L5.JSP MYI-L6.JSP 750 800 850 900 (b) Absorbance Absorbance (a) 700 Color 0.55 0.50 0.45 0.40 0.35 No 1 2 3 4 0.35 0.30 0.30 0.25 File Name MYI-K4.JSP MYI-K5.JSP MYI-K6.JSP MYI-K7.JSP Color 0.25 0.20 0.20 0.15 0.15 0.10 0.10 0.05 0.05 0 0 200 250 300 350 400 450 500 550 600 Wavelength (nm) 650 700 750 800 850 900 200 250 300 350 400 (c) 450 500 550 600 Wavelength (nm) 650 700 (d) 1.00 0.95 0.90 0.85 0.80 0.75 0.70 0.65 Absorbance 0.60 0.55 0.50 0.45 0.40 0.35 0.30 No 1 2 0.25 File Name MYI-K8.JSP MYI-K9.JSP Color 0.20 0.15 0.10 0.05 0 200 250 300 350 400 450 500 550 600 Wavelength (nm) 650 700 750 (e) Figure S1. The normalized UV-Vis spectra of compounds. 800 850 900 750 800 850 900