LAB_Dilutions_Standard Curve
advertisement

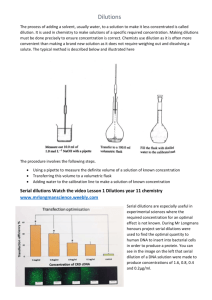
Pipetting Dilutions and Using a Spectrophotometer to Generate a Standard Curve Introduction In this lab we will be generating data using the basic techniques of pipetting using a graduated pipet to prepare a standard curve from dilutions made from a standard solution. Using a known standard solution we can make a series of dilutions and then generate a standard curve. Generating a standard curve gives chemists, biologists and environmental scientists, for example, the ability to interpolate and extrapolate data to determine the concentration of an unknown solution. The spectrophotometer is the instrument used to generate the data and designed to measure the amount of light absorbed by a solution. Using the spectrophotometer we can quantitatively measure absorbance and this information can be used to determine the concentration of the solution. During lab you will be evaluated on the application of skills acquired in the use of: Graduated pipets to measure variable volumes Organizing and generating data Proper use of a spectrophotometer After lab, you will write a laboratory report following the Laboratory Report Guidelines that assesses your ability to: Interpret data Use a standard curve to interpolate and extrapolate data. Use statistics to evaluate the reliability of the data Write scientifically following the conventions within the scientific community for communicating a scientific study in writing. The Laboratory Report Scoring Rubric will be used to evaluate the ability to write scientifically. Follow the scoring rubric to ensure you have included all the required components of a laboratory report. As you are writing, evaluate the quality of what you are writing for both content and the use of collegelevel writing to express your ideas. Pre Lab Activities 1. 2. 3. 4. 5. Review the SOP on the use of graduated pipets. Review the SOP on the use of micropipettes. Review the SOP on the use of spectrophotometers. Review information on how to make a dilution. Review the PowerPoint: Excel- Preparing a Standard Curve. 6. Read the article by Chan, C.C., Lam, H., Zhang, X.M., (2010), Practical Approaches to Method Validation and Essential Instrument Qualification, John Wiley and Sons. http://onlinelibrary.wiley.com/book/10.1002/9780470630716 Rev2Aug2014 Pipetting Dilutions and Using a Spectrophotometer to Generate a Standard Curve Laboratory Procedures The laboratory technician will: Prepare a dilution using a graduated pipet Use a spectrophotometer to generate data Calculate the concentration of a dilution Use Excel to create a standard curve for each dilution set from spectrophotometer data A. Making a Dilution Materials: 20ml of 1.00x10-4 M bromcresol green standard solution Distilled H2O Two Mohr (graduated) pipets and a pipet bulb 5 test tubes (20ml volume) Procedure: 1. Properly label 5 test tubes as 1/10, 2/10, 3/10, 4/10 and 5/10. 2. Obtain two clean 10ml Mohr pipets and bulbs. One will be used for pipetting the standard solution and the other for pipetting water. 3. Obtain the 1.00x10-4 M bromcresol green standard solution. 4. Following the SOP for Graduated Pipets, pipet 1ml of the 1.00x10-4 M bromcresol green standard solution in the 1/10 test tube, 2ml in the 2/10 test tube, 3ml in the 3/10 test tube, 4ml in the 4/10 test tube and 5ml in the 5/10 test tube. 5. Using a clean Mohr pipet, complete the dilutions by adding 9 ml of water in the 1/10 test tube, 8ml of water in the 2/10 test tube and continue doing the appropriate dilutions. 6. Carefully mix each dilution using a vortex mixer. Rev2Aug2014 B. Using a Spectrophotometer to Generate a Standard Curve Materials needed to generate a standard curve: Spectrophotometer Dilution series Cuvettes Computer with Excel Procedure: 1. Follow the pre-operation SOP for spectrophotometers. Turn on the spectrophotometer; the lamp inside it will need to warm up. 2. Set the mode to Absorbance and the wavelength to 620nm. AFTER the spectrophotometer has warmed up: For the dilution set perform the following spectrophotometer analysis. Be sure you have an organized way to record data in the notebook. Follow all SOP’s for the proper use of the pipets and spectrophotometer. 1. Pipet 2ml of distilled water into a clean cuvette, place it into the cell compartment of the spectrophotometer with the arrow facing forward. 2. Close the lid and zero/blank the instrument (this subtracts any absorbance from the water allowing only absorbance of the standard at 620nm). 3. Pipet 2ml of the first 1/10 dilution into a clean cuvette and replace the water with the sample in the cell compartment. Close the lid and record the Absorbance reading from the display. 4. Repeat Step 3 for each dilution and record the data in your lab notebook making sure each sample is correctly identified. 5. Calculate the concentration for each dilution made. 6. Using an Excel spreadsheet, enter the data into the spreadsheet and graph the data using an x-y scatter plot. Make sure to label each graph and both axis. Rev2Aug2014