Lactobacillus
advertisement

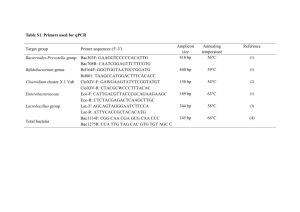
Simultaneous detection and enumeration of Lactobacillus spp., Bifidobacterium animalis subsp. lactis, B. longum subsp. longum and Streptococcus salivarius subsp. thermophilus in commercial fermented milk by DNA biochip and quantitative RT-PCR Speaker: Date: Ya-Yun Chang 2013.06.16 Presenter: Time: Xin-Hong Chen 17:08 ~ 17:30 Number:18 1. Introduction Lactic acid bacteria (LAB) play an important role in food fermentation processes (Vuyst , L. D., 2003; Wood, 1997). Fermented milk starters are often combined with multiple LAB strains with specific characteristics in terms of flavour or health properties. The presence of multiple and closely related species in these products make the differentiation and enumeration of those probiotic strains difficult, due to similarity in growth requirements and overlapping biochemical profiles of the species. Recent reports have shown that the identity of recovered microorganisms does not always correspond to the information stated on the product label (Gueimoned, M., 2004). Plating on media is one of the classic techniques for determining the viability of probiotics, but these techniques are time-consuming and labor-intensive. Therefore, it is necessary to develop effective and less time-consuming methods to identify these bacterias. Molecular techniques can be used to improve the efficiency of detection and enumeration methods. The aim of this study was development of a rapid and easy to diagnosis system based on DNA biochip and quantitative RT-PCR with the ability to differentiate among Lactobacillus spp., Bifidobacterium spp. and S. salivarius subsp. thermophilus in the fermented products. 2. Materials and methods 2.1 Bacterial strains and culture conditions:20 bacterial strains used in this study are listed in Table 1. Bacterial strains were cultivated in 5mL MRS broths or MRS broths containing 0.05% L-cysteine hydrochloride by 24 h incubation at 37℃ under anaerobic condition for further plating or DNA preparation. 2.2 DNA propagation:Genomic DNAs of bacterial were prepared using the Blood & Tissue Genomic DNA Extraction Miniprep System for Bacteria (Viogene) according to the manufacturer’s protocol. 2.3 RNA and cDNA propagation:RNA of bacterial were prepared using the RNeasy Mini kit for Bacteria (Qiagen) according to the manufacturer’s protocol. cDNA of bacterial were prepared using the Omniscript RT kit for Qiagen according to the manufacturer’s protocol. 2.4 PCR primers and probes design:Specific PCR primers and probes used in this study are reported in Table 3 and 4. 2.5 Lactobacillus spp. PCR and multiplex (L. acidophilus(A), B. animalis subsp. lactis(B), B. longum subsp. longum;(B), L. delbrueckii subsp. bulgaricus(L), S. salivarius subsp. thermophilus (S)) PCR followed by direct DNA biochip for the detection of bacterial strains in fermented milks:The PCR primers were labeled at the 5’-end with biotin and then subjected to multiplex PCR for the amplification of biotin-labeled amplicons. These biotin-labeled PCR products were used for oligonucleotide array tests. Oligonucleotide array tests was carried out according to the manufacturer's protocol (Dr. Chip Biotech.). DNA was extracted from the fermented milks followed by PCR and biochip hybridization. 2.6 Enumeration of viable cell numbers in commercial fermented milks by cultural method and comparison of the results with those from the qPCR method:16 commercial Fermented milks(Table 2) purchased from local supermarkets were used as samples. All samples were purchased within the expiration date. After purchasing, these products were stored at 4℃ and Lactobacillus spp., Bifidobacterium spp. and S. salivarius subsp. thermophilus cells were assayed and counted immediately. Total counts of Lactobacillus, Bifidobacterium and Streptococcus species in these products were determined by counting the CFU in 1 mL of the serial dilutions after spreading 100μL of these dilutions, respectively, on MRS agar and MRS agar containing 0.05% L-cysteine hydrochloride. For agar media, cells were incubated at 37 ◦ C for 18 to approximately 24h under anaerobic condition. Total DNA was extracted according to the procedures described earlier. In addition, Lactobacillus spp., Bifidobacterium spp. and S. salivarius subsp. thermophilus determined by qPCR using the SYBR Green PCR Master kit. The dissociation analysis determines the cycle threshold value (ct value) of amplification products generated during PCRs. 3. Results and discussion 3.1 Specificity of the Lactobacillus spp. primers, and biochip:Lactobacillus spp. primer was tested with target bacterial strains listed in Table 1. The primer used in this study generated amplicons with expected sizes(Fig.1). All location of the probes and interpretation of patterns of DNA biochip were demonstrated in Fig.2. 3.2 Specificity of the multiplex PCR method and ABLS biochip:The multiplex PCR indicated that expected amplicons for each target bacteria were produced(Fig.4). All location of the probes and interpretation of patterns of DNA biochip were demonstrated in Fig.5, Fig.6, Fig.7, Fig.8 and Fig.9. 3.3 Direct DNA biochip for the detection of bacterial strains in commercial fermented milks:The positive controls using biotin-labeled primers only were included in the chips. Such positive control gave the assurance of chromogenic reaction for each chip(Fig.2, Fig.3, Fig.5, Fig.6, Fig.7, Fig.8 and Fig.9). Fig.3 also demonstrated that the hybridization patterns are in compliance with designed patterns either from single target or multiple target bacterial strains in fermented milks. In this result, we have shown that the identity of recovered Lactobacillus spp. correspond to the information stated on the product label. 3.4 Comparison of bacterial counts using enumeration method and RT-qPCR in commercial fermented milks(Table.5):16 Fermented milks products available in markets were assayed for the presence of ABLS and the total cell counts of Lactobacillus spp., Bifidobacterium spp. and S. salivarius subsp. thermophilus. Total culturable counts of Lactobacillus and S. salivarius subsp. thermophilus determined with MRS agar plates were 6.09 ± 2.33×107 CFU/mL and total bifidobacteria counts determined with MRS agar containing 0.05% L-cysteine hydrochloride plates were between 6.12 ± 2.67×107 CFU/mL. The viable cell counts and bacteria species for LAB in fermented milks were corresponded to those labeled on the products and the Chinese National Standards (CNS) 3058 standard. 3.5 Recent reports have shown that the identity of recovered microorganisms does not always correspond to the information stated on the product label by 16S rDNA gene(Gueimonde, m., et al., 2004) and 16S rRNA gene(Collado, M.C., et al., 2007) but the high similarities of 16S rDNA sequences among closely related species make it difficult to develop highly specific primers to differentiate species. Recently, several monocopy target genes, including tuf (Ventura and others 2003; Solano-Aguilar and others 2008), recA (Kullen and others 1997), Idh (Roy and Sirois 2001), and hsp60 genes (Zhu and others 2003), revealed high divergence in LAB species and could serve as alternative molecular markers. Therefore, that chromogenic DNA macroarray and RT-qPCR developeds fast methods for the identification, enumeration and discrimination of viability of Lactobacillus spp., Bifidobacterium spp. and S. salivarius subsp. thermophilus mixed in fermented milk products. 4. Conclusion Identification of species is a important issue to be verified for the compliance of fermented milk with the required product specifications in terms of accurate species labelling and, if appropriate, to support health claims that could be associated with added probiotics. In this study shows that DNA biochip and RT-qPCR has great detection and identification for verification of accurate bacterial counts and species labeling in fermented milk. 5. References 1. Vuyst, L. D., et al. (2003). Exopolysaccharide-producing Streptococcus thermophilus strains as functional starter cultures in the production of fermented milks. INT DAIRY J, 13, 707-717. 2. Gueimoned, M., et al. (2004). Viability and diversity of probiotic Lactobacillus and Bifidobacterium populations included in commercial fermented milks. FOOD RES INT, 37, 839-850. 3. Collado, M. C. et al. (2007). Identification and differentiation of Lactobacillus, Streptococcus and Bifidobacterium species in fermented milk products with bifidobacteria. Microbiol. Res, 162, 86-92. 4. Sheu, S. J. et al. (2010). Use of tuf gene-based primers for the PCR detection of probiotic Bifidobacterium Species and enumeration of Bifidobacteria in fermented milk by cultural and quantitative Real-Time PCR methods. J. Food Sci, 75, (8), 521-527.