Electromagnetic Waves (option G)
advertisement

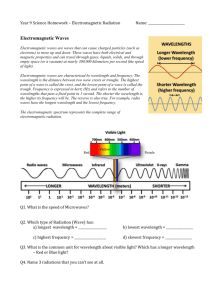
1 Electromagnetic Waves (option G) 1. The nature of electromagnetic (EM) and light sources The origin and creating of electromagnetic wave EM Waves are produced by the accelerated electric charges (when they change speed or direction) . Charged object produces electric field. Accelerating charge produces changing electric field. James Maxwell theory (later confirmed by experiment) found that the changing electric field generate magnetic field. ⇒ Accelerating charge creates both magnetic and electric field. Examples: 1. radio waves antenna: an alternating current produces a radio wave 2. light Light waves have a much higher frequency than radio waves. However, it is not possible to produce light by simply moving a charge up and down very fast (antenna), as it is not possible to change the direction of charge quickly enough. Atoms contain electrons that can exist in different energy levels. When an electron changes from a high energy level to a low one it emits electromagnetic radiation/photon. Energy of the photon equals the electron’s change in energy ΔE. The frequency of the light, f is related to the change in energy ΔE by the equation: where h is Planck’s constant 6.63x 10-34 Js ΔE = hf Light emitted when electron changes from high energy orbit (E3) to low energy orbit (E2) Since there are many electron energy levels in an atom this leads to the emission of light with many different frequencies, each frequency corresponding to a different colour 3. Even higher frequencies Electron energy levels are in the order of 10 eV. This is 10 x 1.6 x10-19 J. Using the formula ΔE = hf an energy change of 10 eV will give rise to light with a frequency of 2.42 x10 15 Hz. However, EM radiation with much higher frequency, over 1020 Hz, does exist. This would need an energy change in the order of MeV, much greater than electron energies. Radiation with such high energy comes from the nucleus. Characteristics of EM waves EM wave is a transverse wave, the electric and magnetic fields oscillate perpendicular to each other and the electromagnetic wave moves in a direction perpendicular to both of the fields. Any electromagnetic wave made up of changing electric and magnetic fields travel through vacuum with the SAME SPEED! speed of light c ≈ 3 x 108 m/s The speed of EM waves is INDEPENDENT of the motion of the source. EM waves are waves, so: c = λf (in vacuum: independent of the frequency of the wave) 2 EM waves carry energy (as any wave does) – example: warmth on the skin while walking outside The energy of a wave is directly proportional to its frequency, but inversely proportional to its wavelength. Short wavelengths are more energetic than long wavelengths. On the other hand energy (and intensity) of a wave is proportional to its amplitude2. The electromagnetic spectrum (EM spectrum) Waves can be classified in terms of their wavelength. Each range of wavelength has a different name, different mode of production and different uses. The regions are not clearly separated, For example, there is considerable overlap between X-rays and gamma rays. Some you can see (visible part), some you can feel - sense (infrared – heat) , some can do damage (ultraviolet), and some could do a lot of harm or could save your life (x-rays, gamma rays). Some will make your life comfortable and very dependent on (microwave oven – radio , TV, cell phones...),... Some animals can “see” light that we can not. Yellow eyelash viper can “see” (sensors) infrared radiation. EM spectrum – the whole range of possible wavelengths ( or frequencies). You should be familiar with the order of magnitude of the frequencies (and wavelengths) of the different regions. Radio waves Radio waves have the longest wavelengths, and lie in the frequency range from 30 Hz to greater than 3000 MHz. Radio waves are produced (among other ways) from an alternating current in a tuned electrical circuit. They are also produced as a piece of adhesive is slowly peeled from a surface, as you can confirm by holding a transistor radio near the tape and listening for pops and snaps coming from the speaker. As they do not penetrate solid materials, radio waves are relatively easily reflected off surfaces and this makes them ideal for communication technology: AM (amplitude modulated) and FM (frequency modulated) radio, television, CB radio, radio microphones and scanning devices in MRI (magnetic resonance imaging). Because of their many uses governments must regulate the bandwidth that can be used in communication devices in order to avoid congestion of the airwaves. Long and medium wavelength radio waves easily diffract around obstacles such as small mountains and buildings, and they can be reflected by the earth’s ionosphere. Therefore, there does not have to be a direct line of sight between the antenna and the receiver and they can be broadcasted over large distances provided the transmitter used is powerful. Short wavelength radio waves are not reflected off the ionosphere in the upper atmosphere but rather pass into space. For this reason, these bands of radio waves are used for outer space and satellite communications. These outer bands are overlapping in the microwave region of the EM spectrum. 3 Microwaves Short-wavelength radio waves 30 cm -1mm; frequencies from 109 Hz – 1012 Hz. Since microwaves have a high frequency they can be used to transfer data: long distance telephone conversations, mobile phone, satellite communication, short-range internet links. Radar consists of short pulses of microwaves. They are used to detect the speed of vehicles by police, and to find distances to aeroplanes and ships. It is a microwave system that guides large airliners into airports. Microwave oven: microwaves of frequency 2450 MHz/10 cm resonate with water molecules, the molecules vibrate and the increased kinetic energy implies an increase in temperature. The heat is therefore generated in the substance itself rather than conducted in from the outside, and this allows food to be cooked rapidly. At short distances from the source, microwave radiation can damage living tissue. They are also used in the analysis of fine details concerning atomic and molecular properties of matter . Infrared EM waves with frequencies just below that of red light (1012 Hz – 4.3 x1014 Hz; λ: 1mm – 700 nm) These waves can be felt as heat on our skin but cannot be seen with our eyes. Infrared rays are often generated by the rotations and vibrations of molecules of the hot objects. In turn, when infrared rays are absorbed by an object its molecules rotate and vibrate more vigorously, the internal energy of the body increases resulting in an increase in the object’s temperature. Infrared is used in TV remote controls, optical communications, and physical therapy. Most remote controls operate on a beam of infrared light with a wavelength of about 1000 nm. This infrared light is so close to the visible light and so low in intensity that it cannot be felt as heat. It is also used in night vision binoculars to see warm objects in the absence of visible light. Thermo grams – photographs made with infrared radiation. Computer translates intensity and frequency of the radiation into colors that we can recognize. Warmer colors indicate higher temperatures. Left – cat’s head – warmest red, coolest blue – Right – Atlantic Ocean off the coast of the North America – warmest swirling red streak is Gulf Stream. They can also identify environmental problems. Because this radiation is scattered by small particles in the atmosphere, they can be used in haze photography. It is also employed in the identification of the molecular structure of many organic compounds/ vibrational spectroscopy. Visible light Visible light is in the range of frequencies that receptors in our eyes are sensitive to EM Colour Wavelength radiation between about 400 nm to 700 nm. Our brains respond to different violet 380—450 nm frequencies by seeing them as different colours: red is the lowest frequency and blue blue 450—495nm the highest. Humans have evolved with vision most sensitive to the wavelengths that green 495—570nm are strongest, the most intense from the Sun. In addition to that, although our Sun yellow 570—590 nm emits all frequencies, most of the frequencies are absorbed when the radiation passes orange 590—620 nm through the atmosphere. red 620—750 nm The eye’s sensitivity is maximum at λ ≈ 560 nm (yellow-green) Aliens from another planets, with a Sun with different temperature would have the center of sensitivity at different wavelengths than ours. It’s also true that if we used radio to see then we would have to have antennae instead of eyes and that wouldn’t look very attractive. Use: optics and optical instruments 4 Ultraviolet EM waves with frequencies just above that of violet light— 7.5x1015 Hz to 1017 Hz (λ: 10-7 m - 10-9 m) Ultraviolet radiation is produced by high energy electron transitions. Ultraviolet cannot be seen but does cause the emission of visible light from some substances. This is why white clothes glow when illuminated by ultraviolet disco lights. Although these rays are invisible, they often make their presence known by causing sun tans with moderate exposure. More prolonged or intense exposure can have harmful consequences, including an increased probability of developing a skin cancer. Fortunately, most of the UV radiation that reaches Earth from the Sun is absorbed in the atmosphere by ozone (03) and other molecules in the stratosphere. At least for now. A significant reduction in the ozone concentration in the stratosphere could result in an unwelcome increase of UV radiation on Earth’s surface. UV radiation has the ability to ionise atoms and this is the reason why ozone is produced in the atmosphere. This ozone is capable of killing bacteria and therefore it can be put to good use in the sterilisation of many objects. The atoms of many elements emit UV radiations that are characteristic of those elements, and this quality allows many unknown substances to be identified. UV radiation can be detected by photography and the photo-electric effect. Furthermore, certain crystals fluoresce when they absorb UV radiation, and this is put to use in washing powders to make the “whites look whiter”. X-rays f: between about 1017 Hz to 1020 Hz, It can be generated by the rapid deceleration (stopping) or deflection of fast-moving electrons when they strike a metal target or other hard objects. X-rays can penetrate different materials to different degrees, depending on the frequency/energy and the material itself. For example, X-rays can easily penetrate skin and flesh containing mainly the lighter atoms carbon, hydrogen and oxygen, but are less able to penetrate dense material such as bone containing the heavier element calcium. Because X-rays are detected by photography, the photographic plate placed beneath the body can be used to identify possible bone fractures. X-rays are ideal for identifying flaws in metals. They are also used in CAT scans (computerised axial tomography). Because tissues absorb X-rays differently, when a body is scanned, the internal organs and tissues can be identified from the analysis of the images produced by the computer. X-radiation can ionise gases and cause fluorescence. Because X-rays produce interference patterns when they interact with crystals in rocks and salts, the structure of these regular patterns of atoms and molecules can be determined by this process of X-ray diffraction. X-rays can damage living cells and continued use and exposure to X-rays is discouraged. Radiologists who work in X-ray departments always stand behind lead-lined walls when an X-ray is being taken. On the other hand, some types of diseased cells are damaged more easily than are healthy cells. Therefore, if X-rays are carefully controlled, they can be used to destroy cancerous cells, as is the case with the use of certain lasers in radiation therapy. Gamma radiation (γ) The gamma radiation region of the EM spectrum overlaps with the X-ray region and their use in cancer therapy overlaps with the last statement made about X ray use in radiotherapy. These high frequency rays are highly penetrating and are produced by natural and artificial radioactive materials. As such, gamma rays will pass through metres of air and need large thicknesses of concrete or lead to absorb them in order to protect humans from danger. Gamma radiation can be detected by an ionisation chamber as found in a Geiger-Müller counter. More recently they are used to kill microorganisms in food. If you see a label in the grocery store that tells ”irradiated food” you will now know that it has been exposed to γ rays from cobalt-60 from 20 to 30 min. NASA has irradiated astronauts’ food since the 1960s. 5 EM radiation and health When electromagnetic radiation is absorbed by human tissue the effect is dependent on the wavelength. Radio — microwave When radio waves are absorbed by the body, they cause a slight heating, but do not change the structure of the cells. There seems to be no physical reason why they should cause illness, but cases of illness have been attributed to closeness to a powerful source of radio waves such as a radio antenna. The higher frequency of microwaves used in mobile phone communication means that the heating effects are greater but the power of the signal is weak. There is some evidence that a mobile phone held close to the brain for a long period of time might cause some damage. There is however, significant risk for people dependent on electronic devices such as pacemakers, that interference from strong sources of radio signals can result in malfunction. IR The heating effect caused by infrared radiation is significant: exposure to JR can result in burns but low levels of IR cause no harm. Light High powered sources of visible light, such as lasers, can damage the eyes and burn the skin. UV Exposure to ultraviolet radiation triggers the release of chemicals in the skin that cause redness and swelling. The effect is rather like a burn, hence the name sunburn. UV radiation can also change the structure of the skin’s DNA leading to skin cancer. X-ray and γ ray Both X-rays and y (gamma) rays have enough energy to remove electrons from atoms; this is called ionization. When radiation ionizes atoms that are part of a living cell it can affect the ability of the cell to carry out its function or even cause the cell wall to be ruptured. If a large number of cells that are part of a vital organ are affected then this can lead to death. To prevent this there are strict limits to the exposure of individuals to these forms of radiation. The interaction of EM radiation with matter When EM radiation is produced, changing electric and magnetic fields spread out in three dimensions from the source: we say that the wave is transmitted through the medium. The intensity of a wave is the power per unit area, as the wave becomes more spread out its intensity becomes less. If the power (of the source) in the whole wave is P then at a distance r this power is spread over a sphere of area 4πr2 . The intensity, I, at a distance, r, is therefore I= P 4πr2 So, there is an inverse square relationship Radio waves spread out in a sphere centred on the source. I 1 r2 As the wave spreads out it interacts with atoms of the medium. First, let's look at the general properties of light interacting with matter. When light strikes an object it will react in one or more of the following ways depending on whether the object is transparent, translucent, opaque, smooth, rough, or glossy: It will be wholly or partly transmitted . It will be wholly or partly reflected. It will be wholly or partly absorbed. 6 How a receiving material responds when light is incident upon it depends on the frequency of the light and the natural frequency of electrons in the material and vibrational natural frequencies of the molecules. If the frequency of light matches one of the natural frequencies of the material light will be absorbed and converted into vibrational energy of the electrons/molecules - thermal energy. If the frequency of light waves do not match natural freq., the electrons in the atoms of the object still begin vibrating but not with great amplitudes. If the object is transparent for particular frequency vibrations of the electrons are passed on to neighboring atoms through the bulk of the material and reemitted on the opposite side of the object. If the object is opaque, the electrons of atoms on the material's surface vibrate for short periods of time and then reemit the energy as a reflected light wave. Such frequencies of light are said to be reflected. This is why microwaves are absorbed by water molecules; IR radiation is absorbed by atoms in solids; and UV radiation is absorbed by the ozone layer. However, the energy in an X-ray is too high to excite an atomic electron and it can pass through most solids. Transmission Transmission takes place when light passes through an object; the object, in this case, is said to be transparent. Light striking the surface of an object straight on (that is, at normal incidence) will pass through without refraction. But light striking at any other angle will be refracted as well as partially reflected: Transparent materials are clear (can be seen through). Reflection The colour of objects can be explained in terms of reflection and absorption of different wavelengths of light. If a mixture of red, blue and green light is shone onto a blue object the red and green is absorbed but the blue is reflected. Law of reflection: r = i Refraction EM radiation travels at different speeds in different mediums. When a wave passes from one medium to another the change in speed causes its direction to change. This explains why a ray of light bends when it passes through a block of glass. When the light passes from one material into other at an angle, the light beam is refracted according to Snell's law n1 sin1 = n2 sin2 Refractive index n of a medium (substance) is defined as the ratio of the speed in a vacuum, c, to the speed of light in a material through witch it passes, v: n = c/v As v can never be greater than c, refractive index is always greater than 1.00. The refractive index of air is usually taken as 1.0, although its true value is 1.0003. The frequency, f, of the wave remains constant when the light passes through substance. Thus, if the velocity, c, is reduced on passage through a substance, the wavelength, λ, must also decrease. Dispersion (Chromatic dispersion) the speed of light through a material varies slightly with the frequency of the light. Each wavelength is refracted at a slightly different angle when passing through a material at an angle. The longer the wavelength, the smaller index of refraction. nred < nblue , red light is refracted less than blue light This is why rainbows are produced when white light passes through a prism. Synthesis of white light: the spectra are combined together and they appear as white colour. 7 Scattering Scattering takes place when light is scattered in all directions while passing through material. The object is said to be translucent. Translucent materials allow light to pass through them only diffusely giving diffuse images (frosted glass). The colours of our world If the Earth had no atmosphere, what color would the sky be? If the Earth had no atmosphere, the sun’s light would travel directly from the Sun in a straight line towards our eyes and we would see the Sun as a very bright star (white) in sea of blackness. With atmosphere? Why is the sky blue? When the white light hits a molecule in the air that has the resonant (natural) frequency equal to the some frequency in the white light, the resonance occur. If the molecule that was hit was in the solid or liquid it would make many collisions with other molecules and give up its energy in the form of heat. There is nothing to hit in the atmosphere. So the molecule will reemit in different direction. Scattering is the process of absorption of a light wave by an atom followed by radiation in different direction. The reemitted light is sent in all directions. It is scattered. Tiny particles (The nitrogen and oxygen molecules...) in the air scatter high frequencies and large particles (CO 2 ...) scatter low frequencies of light. Scattering of violet and blue frequencies of sunlight in all directions is what gives the sky its blue color. A clear cloudless day-time sky is blue because molecules in the air (the nitrogen and oxygen molecules...) scatter blue light from the sun more than they scatter red light. Blue light is scattered all around the sky. Whichever direction you look, some of this scattered blue light reaches you. Since you see the blue light from everywhere overhead, the sky looks blue. Although violet light is scattered more than blue, our eyes are more sensitive to blue, so we see a blue sky. If the Earth had atmosphere without small particles, what color would the sky be? Light travels through space in a straight line as long as nothing disturbs it. As light moves through the atmosphere, it continues to go straight until it bumps into a bit of dust or a gas molecule. Then what happens to the light depends on its wave length and the size of the thing it hits. Let’s imagine for the moment that there are no small particles. When light (visible) hits these large particles, it gets reflected in different directions. The different colors of light are all reflected by the particle in the same way. The reflected light appears white because it still contains all of the same colors. Whichever direction you look, some of this reflected light reaches you. Since you see the white light from everywhere overhead, the sky looks white. If there were no small particle the sky would be white!!!!!! Why Sunsets Are Red? As the day progresses and the sun is lower in the sky, the path of the light through the atmosphere is longer before it gets to you; more short wavelength violet, blue and greens are now scattered from the sunlight. As the path through atmosphere is greater there is more chance for the blue light to scatter multiple times, less chance to reach your eye. When we look towards the sun at sunset, we see red and orange colours because the blue light has been scattered out and away from the line of sight. 8 Sources of light Light is produced when atomic electrons change from a high energy level to a low one. Electrons must first he given energy to reach the high energy level. This can be achieved in a variety of ways. The light bulb A light bulb consists of a thin wire filament enclosed in a glass ball. When an electric current flows through the filament, energy is transferred to the filament. This causes the filament to get hot and electrons to become excited (lifted to a higher energy level). Each time an excited electron falls back down to its low energy level a pulse of light is emitted, and these pulses are called photons. The glowing filament of a light bulb. The discharge tube A discharge tube is a glass tube containing a low pressure gas. A high potential difference created between the ends of the tube causes charged particles in the gas to be accelerated. When these fast moving particles interact with the other gas atoms they excite atomic electrons into high energy levels. When the electrons become deexcited light is emitted. The fluorescent tube As you can see from the photograph of the discharge tube containing mercury vapour, it does not produce much light. However, a large amount of radiation in the UV region is emitted. This is invisible to the human eye, but if the inside of the tube is coated with a substance that absorbs UV radiation and gives out visible light (fluorescence) then this invisible radiation is converted to light. The result is a much brighter light. This is the principle behind the common strip light, properly called a fluorescent light. The amount of light from a discharge tube containing mercury vapour is low but the UV radiation is high. Comparison of light from a light bulb, discharge tube and fluorescent tube 9 The laser - Light Amplification by Stimulated Emission of Radiation Spontaneous Emission: An atom in excited state can return to the lower state spontaneously. LED, lamp… Stimulated Emission: Stimulated emission is a very uncommon process in nature but it is central to the operation of lasers. Suppose an electron is already in an excited state and a photon comes along with energy equal to the difference in the energy between the excited state and the ground state of the atom or molecule. What will happen is that the photon will stimulate the electron to fall into the lower energy state emitting a photon which is in phase and in the same direction as original photon. This is resonance process, called stimulated emission. One photon interacting with an excited atom results in two photons coherent photons (identical and in phase) Such waves will constructively interfere, leading to a more intense wave. Population inversion The production of laser light relies on a process that promotes (or pumps) a large number of electrons to a higher energy level. When a sizable population of electrons resides in upper levels, this condition is called a "population inversion". It is necessary to create a population inversion for laser action to occur. Some atoms will undergo spontaneous emission, and the resulting photons cause other atoms to undergo stimulated emission, leading to a chain reaction. The resultant light is composed of one frequency, very intense, and coherent. To have inverse population it is important that higher energy level is metastable state – excited state with longer lifetime, so the electrons can remain in this state for a longer period before they decay to the ground state. Finding substances in which a population inversion can be set up is central to the development of new kinds of laser. The first laser used synthetic ruby a material with chromium ions with metastable state that are able to stay excited for some time after excitation. Electrons are first pumped up to the higher level by flash of light. Some of excited ruby atoms then start to de-excite. This light can then interact with other chromium ions that are in the metastable levels causing them to emit light of the same wavelength by stimulated emission. As each stimulating photon leads to the emission of two photons, the intensity of the light emitted will build up quickly. This is a cascade process. The result is an amplification of the light, hence the name LASER (Light Amplification by the Stimulated Emission of Radiation). The tube flashes, pumping the ruby atoms into the high level. As each photon passes an excited ruby atom it de-excites and another photon is emitted. This is called stimulated emission of radiation because the atoms are being stimulated to give out radiation by the passing photon. MASER Microwave amplification by stimulated emission of radiation LASER Light amplification by stimulated emission of radiation Both apply the same principles, and only differ in the frequency range in which they operate. 10 The theoretical basis of the laser was first proposed by Albert Einstein in 1915. The first design was proposed by Townes and Schawlow in 1958, and the first working laser was produced by Maiman in 1960. Even for a time thereafter, there was very little interest in the applications of laser beams. However, in the past 20 years their applications have become extensive in technology, industry, medicine and communications. Originally viewed as a curiosity, lasers now are found in a wide variety of applications, including • surgery - malignant tissue absorbs laser radiation more strongly than does healthy tissue. Laser beams of a specific wavelength, pulse rate, power density and energy dose are chosen to suit the absorption properties of the particular tumour - as laser therapy to correct the optical properties of the lens of the eye • precision cutting tools, CDROM readers, supermarket checkout stand scanners, and holograms • In military operations - the success of laser guidance systems in weapons • Scientists use lasers to produce a superheated gas that we call plasma. It is hoped that research in this area may one day make nuclear fusion reactors commercially viable. Monochromatic A monochromatic source of radiation is one that has a extremely narrow band of frequencies or extremely small narrow wavelength band (or colour in the case of visible light). Most sources of light emit many different wavelengths. Laser light is monochromatic. Light sources giving many wavelengths are white. Unlike other light sources, each photon of laser light has the same wavelength; this means the laser is a single colour or monochromatic. Coherence When a light bulb emits photons, they are emitted randomly in different directions and with different phase because the filament atoms act independently from each other. The light emitted is incoherent. However, in a laser, each photon of light is in phase with all the other photons. Laser light is a coherent and monochromatic source of electromagnetic radiation. Two sources are coherent if they ▪ have the same frequency ▪ maintain a constant phase difference with each other. ◘ Two coherent waves have a phase difference which remains constant over time. Waves that have the same frequency, the same amplitude and constant phase relationship are said to be coherent. As we have seen, the light from a light bulb is emitted randomly so two light bulbs will not be coherent . However, we can make two coherent sources by splitting one source in two, but first we must make one light source as illustrated. Different parts of a filament give out light of different wavelength and phase, but using a narrow slit we can select just one part of the filament. This doesn’t make the source monochromatic but all parts are in phase. 11 INTERFERENCE Interference is the addition (superposition) of two or more waves overlapping that results in a new wave pattern. When two waves of the same type are incident at the same place they add together to give one resultant wave, this is called superposition. The resultant is dependent on the relative phase of the two waves Principle of superposition: When two or more waves overlap, the resultant displacement at any point and at any instant is the vector sum of the displacements of the individual waves at that point: y = y1 + y2 PD = path difference is the difference in distances traveled by waves from two sources to a point P: PD = d2 – d1 Constructive and distractive interference Two coherent waves traveling along two different paths to the same point will ► interfere constructively if there is a difference in distance traveled that is equivalent to a whole number of wavelengths: PD = n λ ► n = 0, ± 1, ± 2, ± 3, … interfere destructively if there is a difference in distance traveled that is equivalent to a half number of wavelengths: PD = (n + ½ ) λ n = 0, ± 1, ± 2, ± 3, … These principles were presented to explain the two-point source interference patterns that are characteristic of Young's experiment and a wavelength measurement. Yet, these principles are more general in the sense that they can explain any physical situation in which waves take two different paths from two coherent sources to the same point. Such coherent waves will undergo interference resulting in interference pattern. ● Real-world examples of two-point source interference So where in this world do we observe two-point source interference? Where can we experience the phenomenon that light taking two paths from two locations to the same point in space can undergo constructive and destructive interference? This is relatively common for homes located near mountain cliffs. Waves are taking two different paths from the source to the antenna - a direct path and a reflected path. It might be a problem if 2d = (n + ½ ) λ for certain frequency. It’s lost. While the interference is momentary (the plane does not remain in a stationary location), it is nonetheless observable. 12 A relatively common demonstration of sound wave interference can be performed with two speakers in a large room. If both speakers are hooked up to the same sound source producing a monotone sound, then a sound interference pattern can be observed within the room. If one were to walk along a line parallel to the line connecting the speakers, there would be clear locations of destructive and constructive interference. At locations of destructive interference, the sound intensity would become weak, perhaps even barely noticeable. At locations of constructive interference, the sound intensity would be amplified. constructive and destructive interference, This effect occurs when two light beams overlap but it can only be observed if the beams are coherent. Why do I not observe bright and dark fringes along my living room wall from the interference of light from two lamps? Light from an incandescent source is emitted with a completely random phase. Although the light from two separate sources (two light bulbs) will interfere, because of the randomly changing phase no permanent points of constructive or destructive interference will be observed since the phase difference between the waves from the two sources is no longer constant i.e., the sources are no longer coherent. There will be no interference patterns. Young ’s double slit experiment This is one of the great classic experiments of physics and did much to reinforce the wave theory of light. The experiment was carried out by Thomas Young in about 1830. The essential features of the experiment are shown When the light passes through the narrow slit it spreads out due to diffraction; this makes it possible to pass the light through two more slits. Filament bulb S0 is turned into a single source using a narrow slit If laser light is used it is already coherent so the first slit is not necessary. The light from two light bulbs will not be coherent, so there will be no interference pattern. SO, he first made one light source by allowing sunlight to fall onto a narrow single slit which now became a point source. This didn’t make the source monochromatic, but all parts were in phase. He got two coherent sources by splitting one source in two. Young observed a pattern of multi- coloured “fringes” in the screen. When he placed a coloured filter between the single slit and double slit he obtained a pattern which consisted of bright coloured fringes separated by darkness with the central one being the bright.– monochromatic fringe pattern. today: laser light is used as source S0. Two waves from two slits will always start the journey with equal phase, so interference pattern – distribution of constructive and destructive interference depends on their path difference ● The geometry of Young’s double slit experiment S1 and S2 two coherent, monochromatic point sources. D - distance from the sources to the screen ~ m d - distance between the slits ~ mm. The waves from the two sources will be in phase at Q and there will be a bright fringe here. What happens at P distance x from Q? we drew the picture so that S1P = AP, so S2 A is path difference. As D >> d and D >> x, θ’ is very small angle → angle S2AS1 is almost 900 so θ’ ≈ θ 13 The condition for a bright fringe at P distance x from center Q is constructive interference. Path difference d sin θ must be a whole number of wavelengths: ► d sin θ = nλ n = 0, ± 1, ± 2, ± 3, … for small angles θ, tan θ = sin θ tan = x/D sin = n λ /d x nλ = D d ● Spacing s between the fringes: s = xn+1 - xn = n+1 λD - nλD d λD d s= d Young actually use this expression to measure the wavelength of the light he used and it is a method still used today. The condition for a dark fringe at P distance x from center Q is destructive interference. Path difference d sin θ must be an odd number of half wavelengths: ► d sin θ = (n + ½ ) λ n = 0, ± 1, ± 2, ± 3, … x λ = n +1 2 d D intensity ● intensity distribution of the fringes on the screen when the separation of the slits is large compared to their width. The fringes are of equal intensity and of equal separation. If the slit separation is decreased, the pattern will spread out. light sensor can measure intensity of light This equal intensity and equal separation pattern breaks down when the separation between slits is not large compared to their with – fortunately we do not investigate that case 14 ● Diffraction grating - increasing the number of slits The intensity of double slit interference patterns is very low but can be increased by using more than two slits. A diffraction gratings is a series of very fine parallel slits mounted on a glass plate. Additional slits at the same separation will not affect the condition for constructive interference. In other words, the angle at which the light from slits adds constructively will he unaffected by the number of slits. When light is incident on the grating it is diffracted at each slit. The slits are very narrow so the diffraction causes the light to propagate as if coming from a point source. To make the geometry simpler we will consider only parallel light rays setting off from the slits at an angle θ. They’ll come together at infinity (distant point) and interfere. Hence if we look at the light through a telescope at angle θ to the grating a bright fringe will be observed. If the path difference, PD, between neighbours is nλ then they will interfere constructively, if (n + ½ ) λ then the interference will be destructive. d sin θ = n λ d sin θ = (n + ½ ) λ bright fringe dark fringe If white light is viewed through a diffraction grating, each wavelength undergoes constructive interference at different angles. This results in a spectrum. The individual wavelengths can be calculated from the angle using the formula d sin θ = n λ greater λ greater angle The addition of further slits at the same slit separation has the following effects; • bright fringes maintain the same separation. • bright fringes become much sharper. • the overall amount of light being let through is increased, so the pattern increases in intensity. In practice, a diffraction grating is stated by its manufacturer to have “X lines per m, or cm, or mm” For example: 600 lines per mm. Then slit separation is: d = (1/600) mm = 1.67 x 10-6 m 15 Problems: 1 Calculate the frequency of light emitted when an electron changes from an energy of 10 eV to 6 eV 2 An atom has electrons that can exist in 4 different energy levels, 10eV, 9eV, 7eV and 2eV. Calculate: (a) the highest frequency radiation that can be produced (b) the lowest frequency radiation. 3 What energy change would be required to produce EM radiation with a frequency of 1x10 18 Hz? 4. What is the wavelength of green light with a frequency of 6x10 14 Hz? Solutions: 1. ΔE = hf ΔE = 4 eV = (4)(1.6x10-19) J = 6.4 x10-19 J h = 6.63x 10-34 Js f = ΔE/h = 0.97x1015 Hz -19 -19 -34 2. highest ΔE = 8 eV = (8)(1.6x10 ) J = 12.8 x10 J h = 6.63x 10 Js f = ΔE/h = 1.93x1015 Hz -19 -19 -34 lowest ΔE = 1 eV = (1)(1.6x10 ) J = 1.6 x10 J h = 6.63x 10 Js f = ΔE/h = 0.24x1015 Hz -34 18 -16 -16 -19 3. ΔE = hf = (6.63x 10 )( 1x10 ) = 6.63x10 J = 6.63x10 /1.6x10 eV = 4.1x103 eV 4. c = λf λ = c/f = 3x108/6x1014 λ = 500 nm Problems: Use the spectrum to find out what type of radiation the following wavelengths would be and calculate their frequency: 4. 430nm 5. 3.75m 6. 10 μm 7. 1nm Problems: 8. If a light bulb emits 50 W of light what will its intensity be at a distance of 10 m? 9 If intensity of the radiation from the Sun reaching the Earth’s atmosphere is 1400 W/m 2 and the Sun is 146 x 109 m from the Earth, calculate the power of the Sun.