15 N-edited Sequences
advertisement
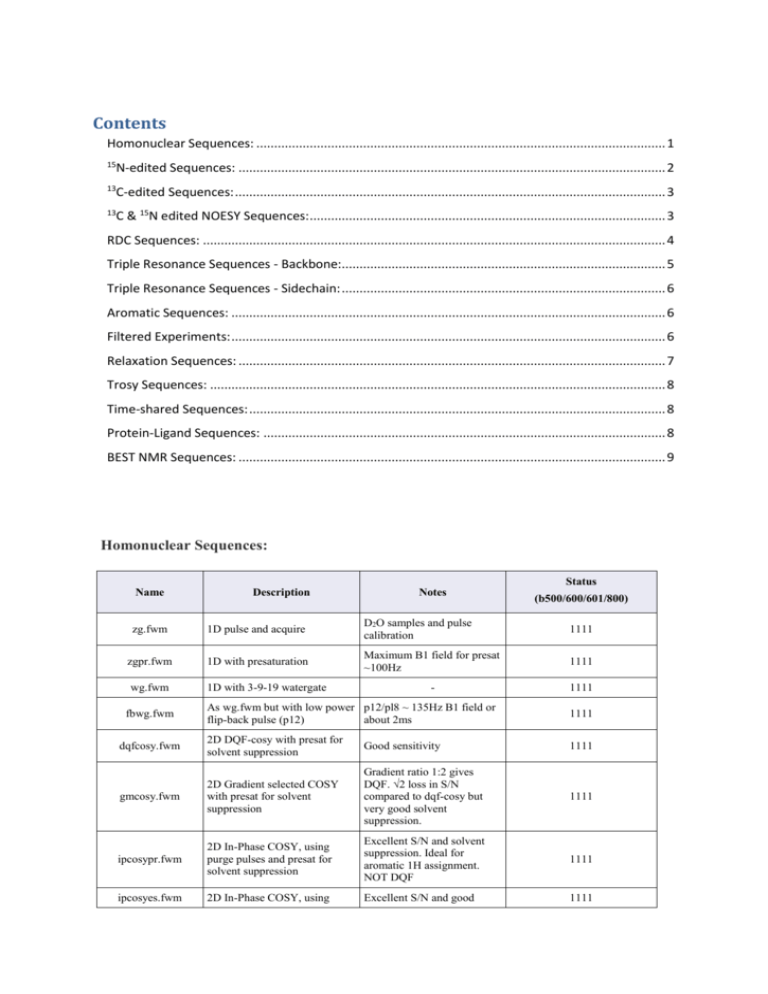
Contents
Homonuclear Sequences: ................................................................................................................... 1
15
N-edited Sequences: ........................................................................................................................ 2
13
C-edited Sequences: ......................................................................................................................... 3
13
C & 15N edited NOESY Sequences: .................................................................................................... 3
RDC Sequences: .................................................................................................................................. 4
Triple Resonance Sequences - Backbone:........................................................................................... 5
Triple Resonance Sequences - Sidechain: ........................................................................................... 6
Aromatic Sequences: .......................................................................................................................... 6
Filtered Experiments: .......................................................................................................................... 6
Relaxation Sequences: ........................................................................................................................ 7
Trosy Sequences: ................................................................................................................................ 8
Time-shared Sequences: ..................................................................................................................... 8
Protein-Ligand Sequences: ................................................................................................................. 8
BEST NMR Sequences: ........................................................................................................................ 9
Homonuclear Sequences:
Status
Name
Description
Notes
(b500/600/601/800)
zg.fwm
1D pulse and acquire
D2O samples and pulse
calibration
1111
zgpr.fwm
1D with presaturation
Maximum B1 field for presat
~100Hz
1111
wg.fwm
1D with 3-9-19 watergate
-
1111
As wg.fwm but with low power p12/pl8 ~ 135Hz B1 field or
flip-back pulse (p12)
about 2ms
1111
dqfcosy.fwm
2D DQF-cosy with presat for
solvent suppression
Good sensitivity
1111
gmcosy.fwm
2D Gradient selected COSY
with presat for solvent
suppression
Gradient ratio 1:2 gives
DQF. √2 loss in S/N
compared to dqf-cosy but
very good solvent
suppression.
1111
ipcosypr.fwm
2D In-Phase COSY, using
purge pulses and presat for
solvent suppression
Excellent S/N and solvent
suppression. Ideal for
aromatic 1H assignment.
NOT DQF
1111
ipcosyes.fwm
2D In-Phase COSY, using
Excellent S/N and good
1111
fbwg.fwm
excitation sculpting for solvent
suppression
solvent suppression.
Attenuation of signals at
solvent from shaped pulses.
prnoesy.fwm
2D noesy with optional presat
-
1111
wgnoesy.fwm
2D noesy with 3-9-19
watergate
-
1111
wgfbnoesy.fwm
2D noesy with 3-9-19
watergate and low power flipback pulse (p12)
Increases S/N on rapidly
exchanging protons.
1111
2D tocsy with optional presat.
prmlev.fwm
wgtocsy.fwm
Uses MLEV-17 spin lock.
2D tocsy with 3-9-19
watergate. Uses DIPSI-2 spin
lock.
-
111X
-
111X
2D tocsy with 3-9-19 watergate
Increases S/N on rapidly
and low power flip-back pulse
exchanging protons
(p12). Uses DIPSI-2 spin lock.
111X
wgdipsi.fwm
2D tocsy with 3-9-19
watergate. Uses DIPSI spin
lock.
Non-clean spin-lock. Good
for large proteins. Good S/N
111X
wgmlev.fwm
About 30% better S/N than
2D tocsy with 3-9-19
DIPSI-2 sequence and ~30%
watergate. Uses MLEV-17 spin more efficient spin-lock. Set
lock.
to "clean" with d20 (22.6*p6)
111X
wgfbtocsy.fwm
15N-edited
Sequences:
Status
Name
Description
Notes
-
(b500/600/601/800)
wghsqc.fwm
HSQC with 3-9-19 watergate
1111
wghsqcd2.fwm
HSQC with 3-9-19 watergate
and 13C decoupling
Uses shaka6 13C decoupling
1111
wgfbhsqc.fwm
As wghsqc but with low power
flip-back pulses
Increases S/N on rapidly
exchanging protons.
1111
sehsqc.fwm
HSQC - sensitivity enhanced.
Solvent suppression via
gradient selection
For proteins of Mr <16KDa
this gives up to √2 increase
in S/N
1111
wgthsqc.fwm
3D-TOCSY-HSQC with 3-9-19 Uses MLEV-17 spin lock
watergate
(see homonuclear sequences)
111X
wgfbthsqc.fwm
3D-TOCSY-HSQC with 3-9-19 As wgthsqc.fwm but with
watergate, low power flip-back excellent solvent suppression
pulses and purge pulses
on the cryoprobe
111X
3D-HNHA with soft watergate
for solvent suppression
Proton carrier jumped so F1
sweep-width can be reduced
1111
SOFAST-HMQC
Collect an HMQC in 3
minutes!
01XX
swghnha.fwm
sfhmqc.fwm
13C-edited
Sequences:
Status
Name
chsqc.fwm
Description
HSQC using gradient selection
for solvent suppression
chsqcsi.fwm
As above but sensitivity
enhanced
cthsqc.fwm
constant-time HSQC using
gradient selection for solvent
suppression
13C
Notes
(b500/600/601/800)
-
1111
Usual S/N impovement for
proteins Mr <16KDa
-
1111
1111
& 15N edited NOESY Sequences:
Status
Name
wgnhsqc.fwm
Description
15N-NOESY-HSQC
with 3-9-19
watergate
15N-NOESY-HSQC
with 3-9-19
wgfbnhsqc.fwm watergate, low power flip-back
pulses and purge pulses
hnh.fwm
cnhsqc.fwm
cnhsqcsi.fwm
15N-HSQC-NOESY-HSQC
13C-NOESY-HSQC
Notes
-
1111
Primarily used for finding noe's between
degenerate amide protons
1001
using gradients Can be done in H2O but D2O is best.
NOE's resolved in F1
13C-NOESY-HSQC
with sensitivity
improvement in
1111
Excellent solvent suppression on
cryoprobes
to suppress solvent
13C
(b500/600/601/800)
1111
Can be done in H2O but D2O is best.
Best water suppression with cryoprobes.
NOE's resolved in F1
1111
chsqcn.fwm
13C-HSQC-NOESY
D2O sequence. NOE's resolved in F3
1111
chmqcn.fwm
13C-HMQC-NOESY
D2O only sequence. Better relaxation
properties than the HSQC sequence.
1111
wgnhco.fwm
3D-NOESY-H(N)CO with 3-9-19
watergate
Like wgnhsqc.fwm but correlates NOE
via C' & HN chemical shift.
1010
Optional flip-back pulse.
1111
As ntrosy.fwm but with 13C decoupling
during F1, F2 & F3. Total acquisition
time restricted to 140ms.
1111
ntrosy.fwm
ntrosyd2.fwm
15N-NOESY-TROSY
with 3-9-19
watergate
15N-NOESY-TROSY
watergate
with 3-9-19
RDC Sequences:
Name
Description
Notes
Status
(b500/600/601/800)
1D deuterium observe
For measuring the
deuterium quadrapolar
splitting in your aligned
sample - follow notes on
the title page
1111
N-TROSY with 3-9-19
watergate - sensitivity
enhanced and
temperature
compensation
Use in combination with
15
N-HSQC to measure 1JHN
0011
IPAP-HSQC for
measuring 1JHN
couplings.
Split with au program
splitipap. WARNING - does
not calculate exp. time
correctly!
1111
mipaphsqc.fwm
IPAP-HSQC for
measuring 1JHN, 1JNC' and
2
JNHA couplings.
Requires 13C labelling and a
Swiss post-doc to measure
the couplings!
1111
Jmodhsqc.fwm
J-modulated-HSQC for
measuring 1JHN
couplings.
Very accurate couplings.
1111
Good for resolving overlap
in moderate size proteins.
Resolved in F1
1111
3D TROSY-HNCO for
measuring 1JHN
couplings.
Good for resolving overlap
in larger proteins. Resolved
in F3
1111
3D HNCO for measuring
1
JCαHαcouplings.
Good for resolving overlap
in moderate size proteins.
Resolved in F2
111
JCHtrhnco.fwm
3D TROSY-HNCO for
measuring
1
JCαHαcouplings.
Good for resolving overlap
in larger proteins. Resolved
in F3
111
JCCahnco.fwm
3D HNCO for measuring
1
JC'Cαcouplings.
Good for resolving overlap
in moderate size proteins.
Resolved in F2
1111
Good for resolving overlap
in larger proteins. Resolved
in F3
1011
Good for resolving overlap
in moderate size proteins.
Resolved in F1
1111
zg2hf4.fm
15
tctrosy.fm
ipaphsqc.fwm
JHNhnco.fwm
JHNtrhnco.fwm
JCHhnco.fwm
3D HNCO for measuring
JHN couplings.
1
3D TROSY-HNCO for
JCCatrhnco.fwm measuring
1
JC'Cαcouplings.
JNChnco.fwm
JNCtrhnco.fwm
3D HNCO for measuring
1
JNC'couplings.
Good for resolving overlap
3D TROSY-HNCO for
in larger proteins. Resolved
1
measuring JNC'couplings.
in F1
1111
Triple Resonance Sequences - Backbone:
Status
Name
Description
Notes
(b500/600/601/800)
3D CBCANH.
Solvent suppression via
cbcanh.fwm
Good sensitivity. Constant
time in F1 & F2
1111
3D CBCA(CO)NH with 3-919 watergate
Constant time in both F2
& F1
1111
3D HNCACB with 3-9-19
watergate and optional low
power flip-back pulses
Better, but not perfect, F1
baseline and lineshape
1111
Modified Bruker code.
1111
gradient selection with an
optional low power flip-back
pulse
cbcaconh.fwm
hncacb.fwm
3D HNCO with 3-9-19
watergate and optional low
wghnco.fwm
power flip-back pulses
3D HNCO with 3-9-19
watergate and low power fliphnco.fwm
back pulse
As per original paper.
Same S/N as wghnco.fwm
but "no" artefacts and
1111
better H2O suppression.
Recommended
wghnca.fwm
hnca.fwm
wghncoca.fwm
3D HNCA with 3-9-19
watergate and optional low
power flip-back pulses
Modified Bruker code.
1111
As per original paper.
3D HNCA with 3-9-19
Same S/N as wghnca.fwm
watergate low power flip-back
but "no" artefacts and
pulses
better H2O suppression.
1111
3D HN(CO)CA with 3-9-19
watergate and optional low
power flip-back pulses
1111
Modified Bruker code.
Triple Resonance Sequences - Sidechain:
Status
Name
Description
Notes
(b500/600/601/800)
hccht.fwm
3D H(C)CH-TOCSY
Can be done in H2O but
should be done in D2O.
Cross peaks resolved in F1
111X
hccht3.fwm
3D HC(C)H-TOCSY
Should be done in D2O.
Cross peaks resolved in F3
111X
hcchc.fwm
3D HCCH-COSY
Can be done in H2O but
should be done in D2O.
1110
Can be done in H2O but
3D (H)CCH-TOCSY
ccht.fwm
should be done in D2O.
111X
Cross peaks resolved in F1
Aromatic Sequences:
Status
Name
Description
Notes
13C-TROSY
for aromatics sensitivity improved
arotrosy.fwm
arocnhsqc.fwm
arohccht.fwm
(b500/600/601/800)
1111
-
13C-NOESY-HSQC
using
gradients to suppress solvent.
Optimised for aromatics. Can be done in H2O
but D2O is best. NOE's resolved in F1
0111
3D H(C)CH-TOCSY for
Optimised for aromatics. Can be done in H2O
but should be done in D2O. Cross peaks
resolved in F1
011X
aromatics
arohccht3.fwm
3D HC(C)H-TOCSY for
aromatics
Optimised for aromatics. Should be done in
D2O. Cross peaks resolved in F3
010X
arohcchc.fwm
3D HCCH-COSY for
aromatics
Optimised for aromatics. Can be done in H2O
but should be done in D2O.
0110
Filtered Experiments:
Terminology:
filter = rejection i.e.
edit = selection i.e.
Name
zgprxf.fwm
C-filter = remove proton signals attached to
13
C-selection = select proton signals attached to
13
Description
1D with presaturation and
15
N filter
C
13
C
13
Notes
C+
13
Not a perfect filter but >
90%
Status
(b500/600/601/800)
1111
Relaxation Sequences:
Status
Name
wghsqcT1.fwm
wghsqcT2.fwm
wghsqcT1Rex.fwm
wghsqcT1Rex1H.fwm
Description
Notes
(b500/600/601/800)
Runs as a pseudo 3D
experiment. Interleaved in
pulse code - run with zg
1111
Runs as a pseudo 3D
experiment. Interleaved in
pulse code - run with zg
1111
Rex
Use au program "interleave"
to run.
1111
1H-1H version of
wghsqcT1Rex
Use au program "interleave"
to run.
1111
Only one relaxation delay
possible
1111
15N
HSQC - for measuring
T1
15N
HSQC - for measuring
T2
15N
HSQC - for measuring
wghsqcT1Rex3d.fwm 3D version of wghsqcT1Rex
wghsqcCC_A.fwm
Cross correlation of dipolar
interactions and CSA in
amide bond
Runs as a pseudo 3D
experiment.
1111
wghsqcCC_B.fwm
Cross correlation of dipolar
interactions and CSA in
amide bond
Runs as a pseudo 3D
experiment.
1111
hsqcnoe.fwm
{1H}-15N HSQC - for
measuring heteronuclear
NOE - Sensitivity enhanced
Runs as a pseudo 3D
experiment. Interleaved in
pulse code - run with zg
Runs for ~0.6 times as long
as "expt" reports
1111
Runs as a pseudo 3D
experiment. Interleaved in
pulse code - run with zg
1110
Runs as a pseudo 3D
experiment. Interleaved in
pulse code - run with zg
1110
Runs as a pseudo 3D
experiment. Interleaved in
pulse code - run with zg
Runs for ~0.6 times as long
as "expt" reports
1111
trosyT1.fwm
trosyT2.fwm
trosynoe.fwm
15N
TROSY - for measuring
T1
15N
TROSY - for measuring
T2
{1H}-15N TROSY - for
measuring heteronuclear
NOE - Sensitivity enhanced
Trosy Sequences:
Status
Name
Description
Notes
(b500/600/601/800)
15N-TROSY
trosy.fwm
with 3-9-19
watergate - sensitivity
enhanced
15N-TROSY
trosyd2.fwm
arotrosy.fwm
ntrosy.fwm
ntrosyd2.fwm
with 3-9-19
watergate - sensitivity
enhanced
13C-TROSY
for aromatics sensitivity improved
15N-NOESY-TROSY
with 3-
9-19 watergate
15N-NOESY-TROSY
with 3-
9-19 watergate
-
1111
13C
decoupling in F1 & F2.
Total acquisition time
restricted to 140ms.
-
1111
1111
Optional flip-back pulse.
1100
As ntrosy.fwm but with 13C
decoupling during F1, F2 &
F3. Total acquisition time
restricted to 140ms.
1100
Time-shared Sequences:
Status
Name
Description
Notes
(b500/600/601/800)
13C
tshsqc.fwm
tshnhacan.fwm
& 15N -HSQC with
sensitivity improvement.
Solvent suppression via
gradient selection
-
0100
Simultaneous acquisition of
3D HNCA & HACAN
-
0100
NOE's resolved in F1
0100
13C
tsnhsqc.fwm
& 15N NOESY-HSQC
with sensitivity improvement.
Solvent suppression via
gradient selection
N.B.: These need special set-up and are kept unavailable to prevent accidents. Please ask if you
want to try them.
Protein-Ligand Sequences:
Status
Name
Description
Notes
(b500/600/601/800)
wgstd.fwm
1D STD experiment 3-9-19
watergate and T1 rho filter
Subtraction done via phase
cycling
1000
wgstd2.fwm
1D STD experiment 3-9-19
watergate and T1 rho filter
Collected as two separate
FID's. Subtraction done post
acquisition.
1000
BEST NMR Sequences:
Status
Name
Description
Notes
(b500/600/601/800)
b_hsqc.fwm
2D HSQC
0001
b_hnco.fwm
3D HNCO
0001
b_hnca.fwm
3D HNCA
0001
b_hncoca.fwm
3D HN(CO)CA
0001
b_hncacb.fwm
3D HNCACB
0001
3D HN(CO)CACB
0001
b_hncocacb.fwm