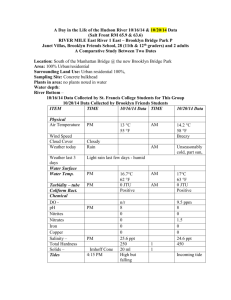
Ph and Dissolved Oxygen tests

Directions for Chemical Tests pH Test
Fill tube to line and add 10 drops of indicator. Mix and compare to colorimeter.
Dissolved Oxygen Test
Fill DO bottle by opening under water and letting the water bubble in. Close under water.
Check for air bubbles when out of the water by inverting the bottle. If bubbles are present, redo the procedure.
1) Add 8 drops of Manganese Sulfate by holding the dropper bottle vertically above the DO bottle (this ensures uniform size of the drops). Do the same with Alkaline Potassium Iodide.
Cap and mix slowly by inverting the DO bottle. A brown gel-like solid should soon be present if oxygen is dissolved in the water.
2) Add 8 drops of Sulfuric Acid. Cap and mix again. (The solid dissolves and there will be an amber colored liquid left).
3) Pour from the DO bottle into the test vial to the 20 mL line.
4) Add 8 drops of starch indicator to the vial. *
5) Fill the syringe (titrator) carefully to avoid air bubbles from the bottle marked Thiosulfate.
Make sure the collar of the plunger is on the zero (0) line of the syringe.
6) Place the titrator in the hole in the cap of the vial and add a drop at a time with swirling to mix. When the solution changes from blue-black to clear, STOP and read the amount of
Dissolved Oxygen in parts per million (ppm) from the titrator.
Conductivity and Temperature
Note – turn the meter on and let the temperature stabilize for a minute or two before using.
A water sample can be collected it the cup and tested or the meter can be place in the water
(only the end of the meter is placed in the water). Read the Temperature and Conductivity on the meter.
*Record the Air Temperature too.
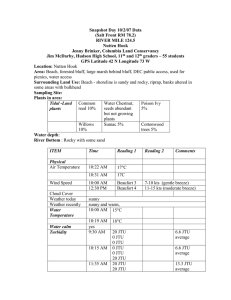
Turbidity
1.
Sample Tube : Fill one of the Turbidity Columns with 50 mL of your water sample. If the black dot at the bottom of the tube is not visible when looking down the column of water, pour out 25 mL of your sample so you are left with 25 mL of the water sample.
2.
Clear water tube : Fill the second Turbidity Column with turbidity-free water (either clear tap water or distilled water) to a volume equal to that of your sample tube (50 or
25 mL).
3.
With the two tubes placed side by side, look down the columns at the black dots and compare the clarity. If the black dots are equally clear in both tubes, the turbidity of your sample is zero. If the black dot in the sample tube is less clear, continue to Step 4.
4.
Shake your Standard Turbidity Reagent vigorously, and then add 0.5 mL to the clear water tube.
Using the stirring rod, stir the contents of both tubes to equally distribute particles. Compare the clarity of the black dots. If the black dot of the sample tube is still less clear than that of the clear water tube, continue adding the Standard Turbidity
Reagent to the clear water tube in 0.5 mL increments until the turbidity of the clear water equals that of your sample (until the black dots in both tubes are equally clear).
5.
50 mL sample: If you used 50 mL of sample in the test, each 0.5 mL addition of the reagent is equal to 5 Jackson Turbidity Units (JTUs).
6.
25 mL sample: If you used 25 mL of sample in the test, each 0.5 mL addition of the reagent is equal to 10 JTUs.
TUBIDITY TEST RESULTS
# of Measured Reagent
Additions
1
2
3
4
5
6
7
8
9
10
15
20
Amount
(mL)
0.5
1.0
1.5
2.0
2.5
3.0
3.5
4.0
4.5
5.0
7.5
10.0
50 mL Sample 25 mL Sample
5 JTU
10 JTU
15 JTU
20 JTU
25 JTU
30 JTU
35 JTU
40 JTU
45 JTU
50 JTU
75 JTU
100 JTU
10 JTU
20 JTU
30 JTU
40 JTU
50 JTU
60 JTU
70 JTU
80 JTU
90 JTU
100 JTU
150 JTU
200 JTU
Alkalinity
Total (T) Alkalinity
1) Fill the test tube to the 5 mL with sample water.
2) Add one BCG-MR Indicator tablet. Cap and swirl to mix until the tablet dissolves. The solution should be blue-green.
3) Fill the titrator with Alkalinity Titration Reagent B and insert in the center hole of the cap.
4) Add the Alkalinity Reagent B a drop at a time with swirling between each drop until the color change from blue-green to purple. (see the Endpoint Color Chart). Record the test result directly from the titrator at the level of the collar of the plunger. Record results as
Total Alkalinity in ppm CaCO
3
.
5) If the color did not change when the titrator was emptied, refill and continue the titration.
Be sure to add the first 200 ppm to the final reading of the titrator.
Chloride
1) Fill the test tube to the 15 mL with sample water.
2) Add one drop of the Phenolphthalein Indicator. If the solution remains clear, go to step 3.
If the solution turns pink, add Sulfuric acid one drop at a time, mixing after each drop, until a pink color appears.
3) Add three drops of Chloride Reagent #1. Cap and swirl to mix. The solution will turn yellow.
4) Fill the titrator with Chloride Reagent #2 and insert in the center hole of the cap.
5) Add the Chloride Reagent #2 a drop at a time with swirling between each drop until the yellow color changes to orange-brown.
6) Read the test result directly from the titrator at the level of the collar of the plunger.
Record the results in ppm Chloride.
Note: If the color did not change when the titrator was emptied, refill and continue the titration. Be sure to add the first 200 ppm to the final reading of the titrator.