Lect-1
advertisement
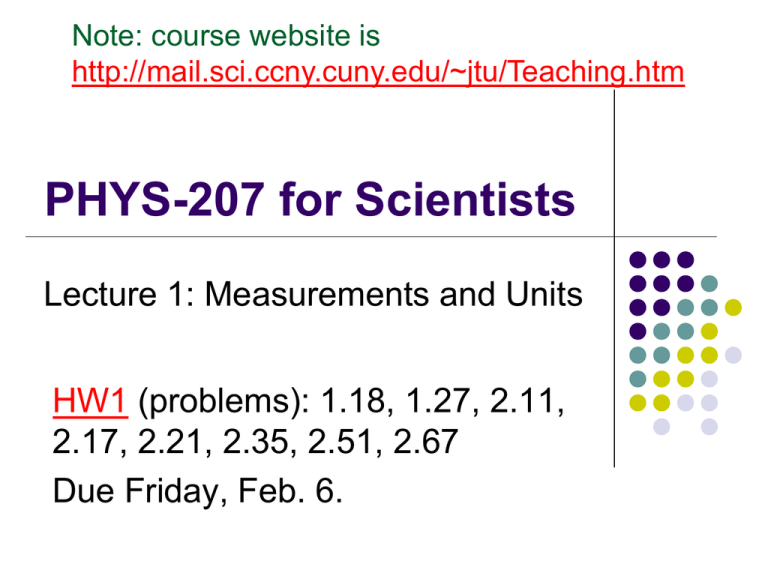
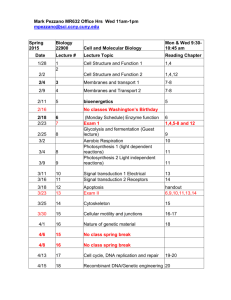
Note: course website is http://mail.sci.ccny.cuny.edu/~jtu/Teaching.htm PHYS-207 for Scientists Lecture 1: Measurements and Units HW1 (problems): 1.18, 1.27, 2.11, 2.17, 2.21, 2.35, 2.51, 2.67 Due Friday, Feb. 6. Note: course website is http://mail.sci.ccny.cuny.edu/~jtu/Teaching.htm PHYS-207 for Scientists Section PP: Honors Instructor: Professor Jiufeng J. Tu Office: MR-330A Marshak Office Hours: Tuesdays, 11:00-2:00 pm E-Mail: jtu@sci.ccny.cuny.edu Requirements Book: Fundamentals of Physics (9th ed.) by Halliday & Resnick, and Walker. Lectures: 417A 9:30 – 10:50 on Tues, Thurs and Fridays in MR- Note: course website is http://mail.sci.ccny.cuny.edu/~jtu/Teaching.htm Labs and Recitations: Fridays, 2:00 – 3:50 in MR-409s (starting on Feb. 6). You have to pass the lab requirements to pass this course. Take the labs (7 in total) seriously since questions on the lab materials will also be in the tests. The Physics Department Lab manual is available on line at http://www.ccny.cuny.edu/physics/introlabman.cfm. Grading Scheme This is how your final grade will be determined: Homework: (~12 of them): 10% Lab Reports: (7 of them): 10% Midterms: (two): 40% Final: 40% Labs: (7) You have to pass the lab requirements to pass this course. Take them serious! Course Scope: Mechanics Kinematics → Dynamics → Thermodynamics 1.2 Measuring things We measure each quantity by its own “unit” or by comparison with a standard. A unit is a measure of a quantity that scientists around the world can refer to. This has to be both accessible and invariable. For example • 1 meter (m) is a unit of length. Any other length can be expressed in terms of 1 meter. A variable length, such as the length of a person’s nose is not appropriate. 1.3 International System of Units The SI system, or the International System of Units, is also called the metric system. Three basic quantities are the length, time, and mass. Many units are derived from this set, as in speed, which is distance divided by time. US Customary System Still used in the US, but text will use SI Quantity Unit Length foot Mass slug Time second Prefixes Prefixes correspond to powers of 10 Each prefix has a specific name Each prefix has a specific abbreviation 1.3 International System of Units Scientific notation uses the power of 10. Example: 3 560 000 000 m = 3.56 x 109 m. Sometimes special names are used to describe very large or very small quantities (as shown in Table 1-2). For example, 2.35 x 10-9 s = 2.35 nanoseconds (ns) Basic Quantities and Their Dimension Dimension has a specific meaning – it denotes the physical nature of a quantity Dimensions are denoted with square brackets Length [L] Mass [M] Time [T] Dimensions and Units Each dimension can have many actual units Table for the dimensions and units of some derived quantities Dimensional Analysis Technique to check the correctness of an equation or to assist in deriving an equation Dimensions (length, mass, time, combinations) can be treated as algebraic quantities add, subtract, multiply, divide Both sides of equation must have the same dimensions Any relationship can be correct only if the dimensions on both sides of the equation are the same Cannot give numerical factors: this is its limitation Symbols The symbol used in an equation is not necessarily the symbol used for its dimension Some quantities have one symbol used consistently For example, time is t virtually all the time Some quantities have many symbols used, depending upon the specific situation For example, lengths may be x, y, z, r, d, h, etc. Conversion of Units When units are not consistent, you may need to convert to appropriate ones Units can be treated like algebraic quantities that can cancel each other out 1.4 Changing units Based of the base units, we may need to change the units of a given quantity using the chain-link conversion. For example, since there are 60 seconds in one minute, 1 min 60 s 1 , and 60s 1 min 60 s 2 min (2 min) x (1) (2 min) x ( ) 120 s 1 min Conversion between one system of units and another can therefore be easily figured out as shown. The first equation above is often called the “Conversion Factor”. 1.5 Length Redefining the meter: In 1792 the unit of length, the meter, was defined as one-millionth the distance from the north pole to the equator. Later, the meter was defined as the distance between two finely engraved lines near the ends of a standard platinum-iridium bar, the standard meter bar. This bar is placed in the International Bureau of Weights and Measures near Paris, France. In 1960, the meter was defined to be 1 650 763.73 wavelengths of a particular orange-red light emitted by krypton-86 in a discharge tube that can be set anywhere in the world. In 1983, the meter was defined as the length of the path traveled by light in a vacuum during the time interval of 1/299 792 458 of a second. The speed of light is then exactly 299 792 458 m/s. 1.5 Length Some examples of lengths Reasonableness of Results When solving a problem, you need to check your answer to see if it seems reasonable Reviewing the tables of approximate values for length, mass, and time will help you test for reasonableness 1.5 Time Atomic clocks give very precise time measurements. An atomic clock at the National Institute of Standards and Technology in Boulder, CO, is the standard, and signals are available by shortwave radio stations. In 1967 the standard second was defined to be the time taken by 9 192 631 770 oscillations of the light emitted by cesium-133 atom. 1.5 Mass A platinum-iridium cylinder, kept at the International Bureau of Weights and Measures near Paris, France, has the standard mass of 1 kg. Another unit of mass is used for atomic mass measurements. Carbon12 atom has a mass of 12 atomic mass units, defined as 1u 1.66053886 x 1027 kg Standard Kilogram 1.5 Density Density is typically expressed in kg/m3, and is often expressed as the Greek letter, rho (r). Example, Density and Liquefaction: Uncertainty in Measurements There is uncertainty in every measurement – this uncertainty carries over through the calculations May be due to the apparatus, the experimenter, and/or the number of measurements made Need a technique to account for this uncertainty We will use rules for significant figures to approximate the uncertainty in results of calculations Significant Figures A significant figure is one that is reliably known Zeros may or may not be significant Those used to position the decimal point are not significant To remove ambiguity, use scientific notation In a measurement, the significant figures include the first estimated digit Significant Figures, examples 0.0075 m has 2 significant figures 10.0 m has 3 significant figures The leading zeros are placeholders only Can write in scientific notation to show more clearly: 7.5 x 10-3 m for 2 significant figures The decimal point gives information about the reliability of the measurement 1500 m is ambiguous Use 1.5 x 103 m for 2 significant figures Use 1.50 x 103 m for 3 significant figures Use 1.500 x 103 m for 4 significant figures Note: course website is http://mail.sci.ccny.cuny.edu/~jtu/Teaching.htm PHYS-207 for Scientists Lecture 1: Measurements and Units HW1 (problems): 1.18, 1.27, 2.11, 2.17, 2.21, 2.35, 2.51, 2.67 Due Friday, Feb. 6.