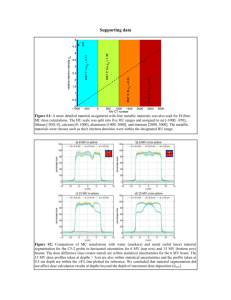
effects of levomilnacipran on the pharmacokinetics of drugs
advertisement

SUPPLEMENTARY MATERIAL Article title: Evaluation of CYP3A4-Based Interactions between Levomilnacipran and Ketoconazole, Carbamazepine or Alprazolam in Healthy Subjects Journal: Clinical Drug Investigation Authors: Laishun Chen, Ramesh Boinpally, Nayra Gad, William M. Greenberg, Julie Wangsa, Antonia Periclou, Parviz Ghahramani Corresponding author: Laishun Chen, Forest Research Institute, Harborside Financial Center, Plaza V, Jersey City, NJ 07311, USA; Email: laishun.chen@frx.com Online Resource 1. Levomilnacipran ER: ketoconazole interaction study: design of Study 1. Sequence I Day(s) Treatment A Day 1 Washout: Days 2–9 Treatment B Days 10–14 Day 15 Days 16–18 Sequence II Day(s) Treatment B Days 1–5 Day 6 Days 7–9 Washout: Days 10–14 Treatment A Day 15 Treatment Blood sampling (h) a Levomilnacipran ER 2 x 40 mg as a single dose (clinic confinement) 0 (predose) 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48, 72 and 96 – No administration of investigational products Ketoconazole 400 mg (2 × 200 mg tablets) (outpatient basis) Co-administration of levomilnacipran ER 80 mg (2 × 40 mg ER capsules) plus ketoconazole 400 mg (2 × 200 mg tablets) (clinic confinement) Ketoconazole 400 mg (2 × 200 mg tablets) (clinic confinement) – Treatment Blood sampling (h) Ketoconazole 400 mg (2 × 200 mg tablets) (outpatient basis) Co-administration of levomilnacipran ER 80 mg (2 × 40 mg ER capsules) plus ketoconazole 400 mg (2 × 200 mg tablets) (clinic confinement) Ketoconazole 400 mg (2 × 200 mg tablets) (clinic confinement) No administration of investigational products – Levomilnacipran ER 2 x 40 mg as a single dose (clinic confinement) 0 (predose) 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48, 72 and 96 0 (predose) 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48, 72 and 96 – 0 (predose) 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48, 72 and 96 – – a Plasma samples were harvested, flash frozen and shipped by overnight courier to the bioanalytical facilities of Forest Research Institute, Inc. ER, extended release 1 Online Resource 2. Levomilnacipran ER:carbamazepine XR interaction study: design of Study 2. Day(s) Treatment A (Period I, Days 1–11) Washout period (Days 12–17) Treatment B (Period II, Days 18–38) Treatment C (Period III, Days 39–49) Treatment D (Period IV, Days 50–53) Treatment Levomilnacipran ER 20 mg for 1 day (AM dose), followed by levomilnacipran ER 40 mg once daily for 3 days (AM dose), followed by levomilnacipran ER 80 mg once daily for 3 days (AM dose), followed by levomilnacipran ER 120 mg once daily for 4 days (AM dose) No administration of investigational products Carbamazepine XR 100 mg twice daily for 4 days, followed by carbamazepine XR 200 mg twice daily for 17 days (AM and PM dosesa) Continuing carbamazepine XR 200 mg twice-daily treatment for the entire period (AM and PM doses) and levomilnacipran ER 20 mg for 1 day (AM dose), followed by levomilnacipran ER 40 mg once daily for 3 days (AM dose), followed by levomilnacipran ER 80 mg once daily for 3 days (AM dose), followed by levomilnacipran ER 120 mg once daily for 4 days (AM dose) Carbamazepine XR 200 mg (AM dose) and carbamazepine XR 100 mg (PM dose) for 1 day, carbamazepine XR 100 mg twice daily (AM and PM doses) for 2 days, followed by carbamazepine XR 100 mg once a day (AM dose) for 1 day Blood sampling (h) b,e On Days 1, 10, 11c: 0 (pre-AM dose) On Day 11c: 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48 and 72 post-AM dose – On Days 18 and 38d: 0 (pre-AM dose) On Day 37d: 0 (pre-PM dose) On Days 39, 48, 49c: 0 (pre-AM dose) On Day 49c: 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48 and 72 post-AM dose On Days 39 and 49d: 0 (pre-AM dose) On Day 48d: 0 (pre-PM dose) On Days 38 and 49d: 0.5, 1, 2, 3, 4, 5, 6, 8, 10 and 12 postAM dose – AM, morning; carbamazepine XR, carbamazepine extended release; levomilnacipran ER, levomilnacipran extended release; PM, evening a For twice-daily doses of carbamazepine XR, the evening dose was administered 12 hours after the morning dose. b Plasma samples were harvested, frozen and sent to Forest Research Institute, Inc., Jersey City, NJ (levomilnacipran and metabolite) or PPD, Inc., Wilmington, NC (carbamazepine and metabolite) for bioanalysis at the end of the study. c Samples for analysis of levomilnacipran and its metabolite. d Samples for analysis of carbamazepine and its metabolite. 2 Online Resource 3. Alprazolam XR/levomilnacipran ER PK interaction study: design of Study 3. Sequence I Day(s) 1 Treatment Alprazolam XR 1 mga 2–9 10 11–13 14–16 17–20 Washout period Levomilnacipran ER 20 mg Levomilnacipran ER 40 mg ODb Levomilnacipran ER 80 mg ODb Levomilnacipran ER 120 mg ODb,c 21 Levomilnacipran ER 120 mg ODb plus alprazolam XR 1 mga 21 As above 22–23 Levomilnacipran ER 120 mg OD Sequence II Day(s) 1 2–4 5–7 8–11 Treatment Levomilnacipran ER 20 mg Levomilnacipran ER 40 mg OD Levomilnacipran ER 80 mg ODb Levomilnacipran ER 120 mg ODb,c 12 Levomilnacipran ER 120 mg ODb plus alprazolam XR 1 mga 12 As above 13–14 15–20 21 Levomilnacipran ER 120 mg ODb,c Washout period Alprazolam XR 1 mga Blood sampling (h)f 0 (predose) 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48, 72 and 96 – 0 (predose) – – On Day 20: 0 (predose) 1, 3, 5, 6, 8 and 12 0 (predose) 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48, 72 and 96d 0 (predose) 1, 3, 5, 6, 8, 12 and 24e – Blood sampling (h)f 0 (predose) – – On Day 11: 0 (predose) 1, 3, 5, 6, 8 and 12 0 (predose) 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48, 72 and 96d 0 (predose) 1, 3, 5, 6, 8, 12 and 24e – – 0 (predose) 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48, 72 and 96 ER, extended release; OD, once daily; PK, pharmacokinetic; XR, extended release; –, no samples taken a Fasted state b Given as single or multiple 40 mg capsules c Fasted state on last day d Samples for analysis of alprazolam XR pharmacokinetics e Samples for analysis of levomilnacipran ER pharmacokinetics f Plasma was harvested and frozen and sent to PPD, Inc., Wilmington, NC (alprazolam) or Forest Research Institute, Inc., Jersey City, NJ (levomilnacipran) for bioanalysis at the end of the study. 3 Online Resource 4 Bioanalytical methods Levomilnacipran and N-desethyl levomilnacipran Concentrations of levomilnacipran and N-desethyl levomilnacipran free bases were determined in plasma using a published liquid chromatography–tandem mass spectrometry (LC-MS/MS) method that had been validated for accuracy, precision, linearity and reproducibility [1]. Briefly, plasma samples were mixed with a solution containing internal standards for levomilnacipran and Ndesethyl levomilnacipran. The analytes were extracted using methyl t-butyl ether and the organic phase was evaporated. The dried residue was reconstituted in 2% formic acid in water–methanol (70:30, v/v). The final extract was analysed via high-performance liquid chromatography (HPLC) with MS/MS detection. The instrument response, which is the ratio of levomilnacipran free base product ion peak area to that of its internal standard, was the response used for quantification of levomilnacipran free base. Similarly, peak area ratio of N-desethyl levomilnacipran free base was the response used for quantification of N-desethyl levomilnacipran free base. For the ketoconazole study, the method used to determine the concentrations of levomilnacipran and N-desethyl levomilnacipran free bases was linear over the concentration range of 1 to 200 ng/mL, with a lower limit of quantification (LLOQ) of 1 ng/mL in 100 μL of plasma with dipotassium EDTA as the anticoagulant. The precision (%CV) and accuracy (%bias) for the plasma levomilnacipran standards were 2.8 and ± 1.6 % respectively. The precision and accuracy for the plasma levomilnacipran quality control samples were 6.0 and ± 0.9 respectively. Similarly, the plasma Ndesethyl levomilnacipran standards had a precision and accuracy of 4.2 and ± 2.7 % respectively, while the plasma quality control samples had a precision and accuracy of 7.0 and ± 4.3 % respectively. For the carbamazepine and alprazolam studies, the method used was linear over a concentration range of 1 to 500 ng/mL for both levomilnacipran and N-desethyl levomilnacipran, with an LLOQ of 1 ng/mL in 100 μL of plasma with dipotassium EDTA as the anticoagulant. Carbamazepine and carbamazepine-10,11-epoxide Plasma carbamazepine and carbamazepine-10,11-epoxide concentrations were determined at PPD Bioanalytical (Richmond, VA, USA) using a sensitive LC-MS/MS method that had been validated for accuracy, precision, linearity and reproducibility, and that is proprietary to PPD. 4 The method was linear over a concentration range of 0.0500 to 50.0 μg/mL for both analytes, with LLOQ at 0.0500 μg/mL in 50 μL of plasma with sodium heparin as the anticoagulant. Alprazolam Plasma concentrations of alprazolam were also determined at PPD Bioanalytical (Richmond, VA, USA) using a sensitive LC-MS/MS method that had been validated for accuracy, precision, linearity and reproducibility, and that is proprietary to PPD. A 500 μL sample aliquot was fortified with 50 μL of internal standard working solution. Analytes were isolated through liquid–liquid extraction. The organic phase was evaporated and the remaining residue was reconstituted with 400 μL of reconstitution solution. The extract was further purified through a hexane wash. The final extract was injected and analysed via HPLC with MS/MS detection. The instrument response, which is the ratio of alprazolam product ion peak area to that of its internal standard ([2H5] alprazolam), was used for quantification. The method was linear over the concentration range of 0.25 to 40 ng/mL with an LLOQ at 0.25 ng/mL using 500 μL of human plasma with dipotassium EDTA as the anticoagulant. The precision (%CV) and accuracy (%bias) for the plasma alprazolam standards were 5.0 and ± 3.1% respectively. The precision and accuracy for the plasma alprazolam quality control samples were 4.0 and ± 9.4% respectively. Reference 1. Chen L, Boinpally R, Greenberg WM, et al. Effect of hepatic impairment on the pharmacokinetics of levomilnacipran following a single oral dose of a levomilnacipran extended-release capsule in human participants. Clin Drug Invest. 2014;34:351–9. 5