File - Earth Science
advertisement

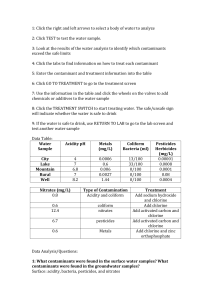
Name_______Justin Powers_________Class_____Date__11/20/14___ Virtual Lab: When Is Water Safe To Drink? Suppose you were hiking along a stream or lake and became very thirsty. Do you think it would be safe to drink the water? In many cases, it wouldn't. Each source of fresh water on or beneath Earth's surface is affected by contaminants. Though the sources of these contaminants are varied, all can make water unfit to drink if they are allowed to increase beyond safe limits. Go to the website: http://www.glencoe.com/sites/common_assets/science/virtual_labs/CT04/CT04.html In this Virtual Lab, you will test a variety of water samples. Then you will determine how to treat the water samples to make them safe to drink Look at the screen to your left, READ and find out about the most common types of water contaminants. Describe what they are and how they might affect water quality. Acidity: It’s measured in pH, and anything lower than 7 is acidic and sodium hydroxide should be added. . Bacteria: When coliform bacteria are present in water, chlorine should be added to clean the water and kill the bacteria. Metals: Chlorine should be added at first to make the metals solid and stick together, and zinc orthophosphate should be added after that to clean even more metals and prevent corrosion. . Nitrates: Carbon should be added to water that contains too many nitrates to get rid of them, and the water should be chlorinated afterwards as well. Pesticides: When pesticides are present, carbon should be added to remove them and then the water should be chlorinated. Objectives: 1. ·Define types of water contaminants. 2. ·Determine which types of contaminants are common to lake water, city water, well water, rural water and mountain water. 3. ·Identify treatments that remove contaminants from drinking water. Procedure: 1. Click the right and left arrows to select a body of water to analyze. 2. Click Test to test the water sample. 3. Look at the results of the water analysis. Identify the “Safe Range” for each category and record this in the data table. 4. Identify which contaminants exceed the safe range. 5. Click the tabs to find information on how to treat each contaminant. 6. Enter the contaminant and treatment information in your data table. 7. Click Go To Treatment to go to the treatment screen. 8. Use the information in the table and click the wheels on the valves to add chemicals or additives to the water sample. 9. Click the Treatment Switch to start treating the water. The Safe/Unsafe Sign will indicate whether the water is safe to drink. 10. If the water is safe to drink, use Return to Lab to go to the lab screen and test another water sample. 11. If the water is unsafe to drink, check your information and treat the water sample again. 12. When you have tested and treated all the water samples, use your completed table to complete the analysis questions. Analysis 1. What contaminants were found in the surface water samples? What contaminants were found in the groundwater samples? Metals were the more common contaminant in the groundwater, and all of the other contaminants, especially coliform bacteria, were found more in the surface water. 2. Why might groundwater and surface water have different contaminants? They are exposed to different chemicals and conditions. 3. Generally, farmers do not farm on the sides of mountains or in remote areas. Industries also do not build factories in these areas. These areas are usually not highly populated by people. What might explain the high nitrate level in the mountain water in this activity? Nitrogen is a key component of soil, and as there is pure soil in the mountains that haven’t been touched by farm, that nitrogen can find its way into the water and bond with the oxygen to make nitrates. 4. What is pH level, what are its characteristics, and how does it contribute to pollution? What chemicals are used in treating low pH levels? pH level is the measure of the acidity of the water, and it goes from 1-14 with 1 being acidic, 7 being neutral, and 14 is the most basic. Critical Thinking-Please read this carefully! Water in an old building tested recently, showed high copper and iron content, and low pH levels. A water reading taken 20 years before, showed low pH levels and only minimal traces of copper and iron. If none of the new buildings on the same street showed signs of metallic contaminants, but all reported lower than normal pH readings, how might these readings be explained? Low pH indicates acidic water, and acids are extremely corrosive to metals. As the building has been around for a while, the acidic water corroded the metals in the pipes and those metals polluted the water. Sample Acidity (pH) Metals (mg/L) Coliform Bacteria (ml) 0 per 100mL Pesticides/Herbicides (mg/L) Nitrates Safe Range 6.5-8.5 City 4 Less than 1.3 mg/L .0006 mg/L Lake 7 Mountain Type of Contamination Treatment Performed Less than .04 mg/L 13/100 mL .00001 mg/L carbofuran Less than 10 mg/L .8 mg/L Acidity and coliform bacteria Sodium hydroxide and chlorine .6 mg/L iron 33/100 mL .0008 mg/L carbofuran .6 mg/L Coliform bacteria Chlorine 6.8 .006 mg/L 0/100 mL .0001 mg/L carbofuran 12.4 mg/L Nitrates Carbon Rural 7 .0027 mg/L copper 0/100 mL .08 mg/L carbofuran 6.7 mg/L Pesticides Carbon and chlorine Well 8.2 1.44 mg/L iron 0/100 mL .0004 mg/L carbofuran .6 mg/L Metals Chlorine and zinc orthophosphate 8