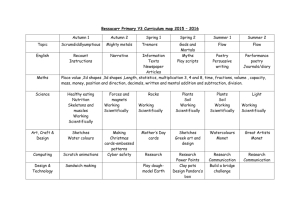
Y7 Science - Castle Manor

Long Term Curriculum Skills and Knowledge Map
Key Stage 3/ Year 7
Subject: Living systems
Science
Curriculum Coverage:
NC 2014 KS3: Structure and function of living organisms: Cells and organisation: cells as the fundamental unit of living organisms, including how to observe, interpret and record cell structure using a light microscope. The functions of the cell wall, cell membrane, cytoplasm, nucleus, vacuole, mitochondria and chloroplasts. The similarities and differences between plant and animal cells. Structure and function of living organisms:
Cells and organisation: the role of diffusion in the movement of materials in and between cells. : Structure and function of living organisms: Cells and organisation: the structural adaptations of some unicellular organisms. The hierarchical organisation of multicellular organisms: from cells to tissues to organs to systems to organisms. The skeletal and muscular systems: the structure and functions of the human skeleton, to include support, protection, movement and making blood cells. The function of muscles and examples of antagonistic muscles. Biomechanics – the interaction between skeleton and muscles, including the measurement of force exerted by different muscles.
Lesson objective
To prepare a slide safely and use a microscope to observe cells
Working Scientifically outcomes:
Evaluate risks; Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety.
Lesson objective
To identify the parts of an animal cell and describe their functions
Working Scientifically outcomes:
Pay attention to objectivity and concern for accuracy, precision, repeatability and reproducibility; Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective
To understand the similarities and differences between plant and animal cells
Working Scientifically outcomes:
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety.
Lesson objective
To collect data and analyse it to explain the connection between surface area and rate of diffusion
Working Scientifically outcomes:
Apply mathematical concepts and calculate results; Present observations and data using appropriate methods, including tables and graphs; Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective
To understand what a unicellular organism is and to describe some examples of how the structures of the organisms enable them to survive
Working Scientifically outcomes:
Present observations and data using appropriate methods, including tables and graphs.
Lesson objective
To order structures to show how living organisms are organised in terms of cells, tissues, organs and systems
Working Scientifically outcomes:
Apply mathematical concepts and calculate results.
Lesson objective
To label a skeleton and to explain the role of the different parts of the bones
Working Scientifically outcomes:
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience.
Lesson objective
To understand how antagonistic muscles provide movement
Working Scientifically outcomes:
Make predictions using scientific knowledge and understanding.
Lesson objective
To successfully build a model of the human arm
Working Scientifically outcomes:
Make and record observations and measurements using a range of methods for different investigations; and evaluate the reliability of methods, suggesting possible improvements. x
X
X
X
X x
X
X
X
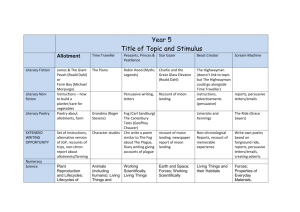
Year 7
P rofessional
R esilient
O ptimistic
U nderstanding
D riven
Subject: The particulate nature of matter
Science
Curriculum Coverage:
NC 2014 KS3: The particulate nature of matter: the properties of the different states of matter
(solid, liquid and gas) in terms of the particle model, including gas pressure. Changes of state in terms of the particle model. Pure and impure substances: diffusion in terms of the particle model.
Matter: Physical changes: Brownian motion in gases; similarities and differences between solids, liquids and gases; diffusion in liquids and gases driven by differences in concentration. Particle model: the differences in arrangements, in motion and in closeness of particles explaining changes of state, shape and density, the anomaly of ice-water transition. Pure and impure substances: the concept of a pure substance; mixtures, including dissolving; the identification of pure substances. Simple techniques for separating mixtures: filtration, evaporation, distillation and chromatography; the identification of pure substances.
Lesson objective:
To recall the three states of matter and the main physical properties of solids, liquids and gases
Working Scientifically outcome:
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions
Lesson objective:
To recall the particle model for a solid, liquid and gas and be able to use it to describe and explain physical properties
Working Scientifically outcome:
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety.
Lesson objective:
To explain expansion using Particle Theory; to make careful observations
Working Scientifically outcome:
Select, plan and carry out the most appropriate types of scientific enquiries to test predictions;
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective:
To recall the particle model of the three states of matter; to recall and explain the changes of state
Working Scientifically outcome:
Present reasoned explanations, including explaining data in relation to predictions and hypotheses.
Lesson objective:
To recall the particle model of a gas and be able to use it to explain observations of experiments involving gaseous diffusion and gas pressure
Working Scientifically outcome:
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience; Present reasoned explanations, including explaining data in relation to predictions and hypotheses.
Lesson objective:
To describe why substances in their solid state are usually more dense than when in their liquid state by using Particle Theory and why water is an anomaly
Working Scientifically outcome:
Make predictions using scientific knowledge and understanding; Select, plan and carry out the most appropriate types of scientific enquiries to test predictions, including identifying independent, dependent and control variables, where appropriate.
Lesson objective:
To recall the definitions of a pure chemical and of a mixture; to understand that a pure chemical has a fixed boiling point whereas a mixture boils over a range of temperatures
Working Scientifically outcome:
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety; Make and record observations and measurements using a range of methods for different investigations.
Lesson objective:
To describe how to carry out filtering and evaporation and explain why these techniques are used to separate a mixture
Working Scientifically outcome:
Select, plan and carry out the most appropriate types of scientific enquiries to test predictions, including identifying independent, dependent and control variables, where appropriate; Make and record observations and measurements using a range of methods for different investigations.
Lesson objective:
To be able to safely carry out a paper chromatography; to be able to interpret a simple chromatogram
Working Scientifically outcome:
Make predictions using scientific knowledge and understanding; Apply mathematical concepts
and calculate results; Use and derive simple equations and carry out appropriate calculations.
X
X
X
X
X
X
X
X
X
X
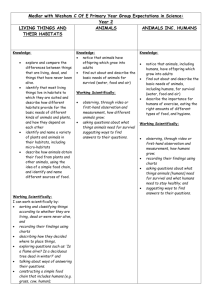
Year 7
P rofessional
R esilient
O ptimistic
U nderstanding
D riven
Curriculum Coverage:
Subject: Forces and motion
Science
NC 2014 KS3: Motion and forces: Describing motion: speed and the quantitative relationship between average speed, distance and time (speed = distance ÷ time).
Forces: forces as pushes or pulls, arising from the interaction between two objects; using force arrows in diagrams, adding forces in one dimension, balanced and unbalanced forces; forces measured in Newtons. Forces: non-contact forces: gravity forces acting at a distance on Earth and in space; Space physics: gravity force, weight = mass x gravitational field strength (g), on Earth g=10 N/kg, different on other planets and stars; gravity forces between Earth and Moon, and between Earth and Sun (qualitative only).
Rubbing and friction between surfaces. Physical changes: similarities and differences, including density differences, between solids, liquids and gases. Forces: forces: associated with deforming objects; stretching and squashing – springs; forces measured in Newtons, measurements of stretch or compression as force is changed; force– extension linear relation; Hooke’s Law as a special case. Forces: associated with rubbing and friction between surfaces, with pushing things out of the way; resistance to motion of air and water; Pressure in fluids: upthrust effects, floating and sinking. Forces: forces as pushes or pulls, arising from the interaction between two objects; using force arrows in diagrams, adding forces in one dimension, balanced and unbalanced forces; Balanced forces: opposing forces and equilibrium: weight held by stretched spring or supported on a compressed surface
Lesson objective:
To demonstrate how speed is calculated and measured in a range of situations
Working Scientifically outcome:
Understand and use SI units and IUPAC (International Union of Pure and Applied
Chemistry) chemical nomenclature.
Lesson objective:
To use distance–time graphs to analyse the movement of objects
Working Scientifically outcomes:
Present observations and data using appropriate methods, including tables and graphs;
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objectives:
To describe the forces between two objects in a range of situations; to represent forces with appropriate force arrows
Working Scientifically outcomes:
Apply mathematical concepts and calculate results; Present observations and data using appropriate methods, including tables and graphs.
Lesson objective:
To describe the difference between mass and weight on the surface of planets and the gravitational forces between planets
Working Scientifically outcome:
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety.
Lesson objective:
To describe the causes and effects of frictional forces and the use of a lubricant to reduce frictional forces
Working Scientifically outcome:
Make and record observations and measurements using a range of methods for different investigations; and evaluate the reliability of methods and suggest possible improvements.
Lesson objective:
To compare materials in terms of density and calculate density from experimental measurements
Working Scientifically outcome:
Understand and use SI units and IUPAC (International Union of Pure and Applied
Chemistry) chemical nomenclature.
Lesson objective:
To examine the behaviour of materials when forces act on them
Working Scientifically outcomes:
Select, plan and carry out the most appropriate types of scientific enquiries to test predictions, including identifying independent, dependent and control variables, where appropriate; Evaluate risks.
Lesson objective:
To describe the effects of upthrust on a floating or sinking object and the effect of drag on motion through a fluid
Working Scientifically outcome
Select, plan and carry out the most appropriate types of scientific enquiries to test predictions, including identifying independent, dependent and control variables, where appropriate.
Lesson objectives:
To examine a range of situations where forces are balanced or unbalanced and describe what will happen to the objects involved
Working Scientifically outcomes:
Apply mathematical concepts and calculate results; Understand and use SI units and
IUPAC (International Union of Pure and Applied Chemistry) chemical nomenclature;
Evaluate risks.
X
X
X
X
X
X
X
X
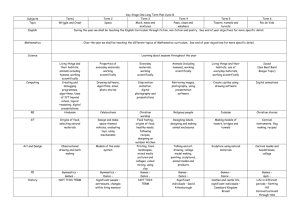
Year 7
X
P rofessional
R esilient
O ptimistic
U nderstanding
D riven
Curriculum Coverage:
Subject: Diet and
Health
Science
NC 2014 KS3: Structure and function of living organisms: Nutrition and digestion: content of a healthy human diet: carbohydrates, lipids (fats and oils), proteins, vitamins, minerals, dietary fibre and water, and why each is needed. Nutrition and digestion: the tissues and organs of the human digestive system, including adaptations to function and how the digestive system digests food (enzymes simply as biological catalysts). NC 2014
KS3: Structure and function of living organisms: Nutrition and digestion: the importance of bacteria in the human digestive system. : The consequences of imbalances in the diet, including obesity, starvation and deficiency diseases. Calculations of energy requirements in a healthy daily diet. Structure and function of living organisms: Health: the effects of recreational drugs (including substance misuse) on behaviour, health and life processes.
Lesson objective:
To design a balanced meal
Working scientifically outcomes
Apply mathematical concepts and calculate results; Present observations and data using appropriate methods, including tables and graphs.
Lesson objective:
To understand what happens during digestion
Working Scientifically outcome:
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience.
Lesson objective:
To understand why enzymes are important for digestion
Working Scientifically outcomes:
Select, plan and carry out the most appropriate types of scientific enquiries to test predictions, including identifying independent, dependent and control variables, where appropriate; Present observations and data using appropriate methods, including tables and graphs.
Lesson objective:
To carry out research the function of bacteria in the digestive system
Working Scientifically outcome:
Pay attention to objectivity and concern for accuracy, precision, repeatability and reproducibility.
Lesson objective:
To carry out food tests and gather data to test a hypothesis
Working Scientifically outcome:
Present reasoned explanations, including explaining data in relation to predictions and hypotheses.
Lesson objective:
To describe the functions of the nutrients
Working Scientifically outcome:
Apply mathematical concepts and calculate results.
Lesson objective:
To understand what is meant by malnutrition and deficiency diseases
Working Scientifically outcome:
Present observations and data using appropriate methods, including tables and graphs.
Lesson objective:
To understand why peoples' diets vary in terms of energy content
Working Scientifically outcome:
Apply mathematical concepts and use SI units.
Lesson objective:
To understand what a drug is and to classify different drugs in general terms legal, illegal, recreational, prescription and over the counter
Working Scientifically outcome:
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Year 7
X
X
X
X
X
X
X
X
X
P rofessional
R esilient
O ptimistic
U nderstanding
D riven
Curriculum Coverage:
Subject: Atoms, elements and compounds
Science
NC 2014 KS3: Atoms, elements and compounds: a simple (Dalton) atomic model; Physics
PoS: Matter: Particle model: atoms and molecules as particles. The Periodic Table: the varying physical and chemical properties of different elements; the principles underpinning the Mendeleev Periodic Table, periods and groups; metals and nonmetals. Atoms, elements and compounds: differences between atoms, elements and compounds. Atoms, elements and compounds: chemical symbols and formulae for elements and compounds. Chemical reactions: combustion, thermal decomposition, oxidation and displacement reactions. Chemical reaction: chemical reactions as the rearrangement of atoms; representing chemical reactions using formulae and using equations.
Lesson objectives:
To recall the names and key properties of the subatomic particles; to be able to recognise and label an atomic model for helium
Working Scientifically outcome:
Understand that scientific methods and theories develop as earlier explanations are modified to take account of new evidence and ideas, together with the importance of publishing results and peer review.
Lesson objectives
To correctly define ‘element’; to understand that elements are grouped by physical properties in the Periodic Table
Working Scientifically outcomes:
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objectives
To recall a definition of a group and a period; to be able to describe how properties change in group 2
Working Scientifically outcomes:
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective:
To define and differentiate between atoms, elements and compounds
Working Scientifically outcomes:
Make and record observations and measurements using a range of methods for different investigations; Make predictions using scientific knowledge and understanding.
Lesson objectives:
To recall a definition of a mixture and a compound; to classify a chemical as a mixture or a compound based on its melting point
Working Scientifically outcomes:
Make and record observations and measurements using a range of methods for different investigations; Evaluate data, showing awareness of potential sources of random and systematic error .
Lesson objective:
To be able to name compounds and interpret names to suggest the elements contained
Working Scientifically outcomes:
Understand and use SI units and IUPAC (International Union of Pure and Applied
Chemistry) chemical nomenclature.
Lesson objectives:
To use the Periodic Table to find the symbol for an element; to give a formula for a simple compound when the name is given; to interpret a formula in terms of how many of each type of atom is present
Working Scientifically outcomes:Understand and use SI units and IUPAC (International
Union of Pure and Applied Chemistry) chemical nomenclature.
Lesson objective:
To correctly define a chemical reaction
Working Scientifically outcomes:
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety; Make and record observations and measurements using a range of methods for different investigations.
Lesson objective:
To recall that atoms in the reactants rearrange to make the products and write word equations
Working Scientifically outcomes:
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions; Understand and use SI units and IUPAC
(International Union of Pure and Applied Chemistry) chemical nomenclature.
Year 7
X
X
X
X
X
X
X
X
X
P rofessional
R esilient
O ptimistic
U nderstanding
D riven
Curriculum Coverage:
Subject: Energy and electromagnetism
Science
NC 2014 KS3: Electricity and electromagnetism: Static electricity: separation of positive or negative charges when objects are rubbed together: transfer of electrons, forces between charged objects; the idea of electric field, forces acting across the space between objects not in contact; Motion and forces: Forces: forces due to static electricity. Current electricity: electric current, measured in amperes, in circuits, series and parallel circuits, currents add where branches meet and current as flow of charge.
Energy changes and transfers: other processes that involve energy transfer: completing an electrical circuit. Potential difference, measured in volts, battery and bulb ratings.
Resistance, measured in ohms, as the ratio of potential difference (p.d.) to current; differences in resistance between conducting and insulating components (quantitative).
Magnetism: magnetic poles, attraction and repulsion; magnetic fields by plotting with compass, representation by field lines; Earth’s magnetism, compass and navigation.
Lesson objectives:
To demonstrate the effects of static electricity and how objects can become electrically charged
Working Scientifically outcome:
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience.
Lesson objectives:
To demonstrate the relationship between current and charge; to use the concept of electric fields to explain the forces that act between charges
Working Scientifically outcome:
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety.
Lesson objectives:
To describe and measure the current in a series circuit
Working Scientifically outcome:
Use appropriate techniques, apparatus, and materials during laboratory work, paying attention to health and safety
Lesson objectives:
To analyse the behaviour of current in parallel circuits confirming its conservation at junctions
Working Scientifically outcome:
Students pay attention to objectivity and concern for accuracy, precision, repeatability and reproducibility; Evaluate data, showing awareness of potential sources of random and systematic error.
Lesson objectives:
To measure the voltage in circuits and find the effect of changing voltages in simple terms
Working Scientifically outcome:
Present observations and data using appropriate methods, including tables and graphs;
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objectives:
To define electrical resistance and find the resistance of sample components; to compare the resistance of components
Working Scientifically outcome:
Apply mathematical concepts and calculate results; Present observations and data using appropriate methods, including tables and graphs; Interpret observations and data, including identifying patterns.
Lesson objectives:
To explain how resistances in a circuit add up; to apply the resistance equation; to describe the operation of a real circuit
Working Scientifically outcome:
Make and record observations and measurements using a range of methods for different investigations; Apply mathematical concepts and calculate results; Evaluate data, showing awareness of potential sources of random and systematic error.
Lesson objectives:
To investigate the effects of a magnetic field on materials and the strength of that field
Working Scientifically outcome:
Select, plan and carry out the most appropriate types of scientific enquiries to test predictions, including identifying independent, dependent, control variables.
Lesson objectives:
To describe the magnetic field around the Earth and the operation of a compass
Working Scientifically outcome:
Understand that scientific methods and theories develop as earlier explanations are modified to take account of new evidence and ideas, together with the importance of publishing results and peer review.
Year 7
X
X
X
X
X
X
X
X
X
P rofessional
R esilient
O ptimistic
U nderstanding
D riven
Curriculum Coverage:
Subject: Reactions
Science
NC 2014 KS3: The Periodic Table: how patterns in reactions can be predicted with reference to the Periodic Table; the Periodic Table: periods and groups; metals and nonmetals; the properties of metals and non-metals. The order of metals and carbon in the reactivity series. The use of carbon in obtaining metals from metal oxides; Chemical reactions: displacement reactions. The Periodic Table: the chemical properties of metal and non-metal oxides with respect to acidity. Energetics: energy changes on changes of state (qualitative); exothermic and endothermic chemical reactions (qualitative).
Chemical reactions: what do catalysts? Physical changes: conservation of material and of mass, and reversibility, in melting, freezing, evaporation, sublimation, condensation, dissolving; the difference between chemical and physical changes. Chemical reactions: combustion, thermal decomposition, oxidation and displacement reactions.
Lesson objective:
To safely investigate the appearance of four different elements to conclude the general properties of metals and non-metals
Working Scientifically outcome:
Students present observations and data using appropriate methods, including tables and graphs; they interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective:
To safely investigate the reaction of some metals with water, acids and oxygen
Working Scientifically outcome:
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience; Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety;
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective:
To safely investigate displacement reactions and describe what is happening
Working Scientifically outcome:
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience; Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety;
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective:
Students should be able to recall that metal oxides are basic and non-metal oxides are acidic. Some students will be able to use their knowledge to make predictions of the outcome of reactions.
Working Scientifically outcome:
Make predictions using scientific knowledge and understanding; Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety; Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective:
To safely investigate the temperature changes in some reactions
Working Scientifically outcome:
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety; Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety; Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective:
To correctly define a chemical change and a physical change; to give examples of chemical and physical changes
Working Scientifically outcome:
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective:
To safely use a variety of methods to monitor a chemical reaction
Working Scientifically outcome:
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety; Make and record observations and measurements using a range of methods for different investigations; and evaluate the reliability of methods suggesting possible improvements.
Lesson objective:
To list the products of combustion for a hydrocarbon and investigate thermal decomposition of metal carbonates
Working Scientifically outcome:
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety; Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Year 7
X
X
X
X
X
X
X
X
X
P rofessional
R esilient
O ptimistic
U nderstanding
D riven
Subject: Genetics and Evolution
Science
Curriculum Coverage:
NC 2014 KS3: Genetics and evolution: Inheritance, chromosomes, DNA and genes: differences between species. The variation between individuals within a species being continuous or discontinuous, to include measurement and graphical representation of variation. Heredity as the process by which genetic information is transmitted from one generation to the next. A simple model of chromosomes, genes and DNA in heredity, including the part played by Watson, Crick, Wilkins and Franklin in the development of the DNA model. The variation between species and between individuals of the same species means some organisms compete more successfully, which can drive natural selection. The variation between species and between individuals, and selective breeding (selective breeding not included in KS3 programme of study but KS4 underpinning). Changes in the environment may leave individuals within a species, and some entire species, less well adapted to compete successfully and reproduce, which in turn may lead to extinction.
Lesson objective:
To understand how scientists classify organisms
Working Scientifically outcome:
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience.
Lesson objective:
To collect variation data and analyse it using charts
Working Scientifically outcome:
Present observations and data using appropriate methods, including tables and graphs;
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective:
To explain why children look like their parents; to identify examples of genetic and environmental variation
Working Scientifically outcome:
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions; Present reasoned explanations, including explaining data in relation to predictions and hypotheses.
Lesson objective:
To understand what DNA, genes and chromosomes are and their function in the body
Working Scientifically outcome:
Understand that scientific methods and theories develop as earlier explanations are modified to take account of new evidence and ideas, together with the importance of publishing results and peer review.
Lesson objective:
To design a model of DNA and understand how its structure was discovered
Working Scientifically outcome:
Understand that scientific methods and theories develop as earlier explanations are modified to take account of new evidence and ideas, together with the importance of publishing results and peer review.
Lesson objective:
Students describe and explain how adaptations help organisms to survive in their environment.
Working Scientifically outcome:
Ask questions and develop a line of enquiry based on observations of the real world, alongside prior knowledge and experience; Present reasoned explanations, including explaining data in relation to predictions and hypotheses.
Lesson objective:
To explain how the process of natural selection occurs
Working Scientifically outcome:
Understand that scientific methods and theories develop as earlier explanations are modified to take account of new evidence and ideas, together with the importance of publishing results and peer review.
Lesson objective:
To be able to outline the steps needed to carry out selective breeding; to discuss the use of genetic modification
Working Scientifically outcome:
Make predictions using scientific knowledge and understanding; Present reasoned explanations, including explaining data in relation to predictions and hypotheses.
Lesson objective:
To understand the causes of extinction
Working Scientifically outcome:
Understand that scientific methods and theories develop as earlier explanations are modified to take account of new evidence and ideas, together with the importance of publishing results and peer review; Present reasoned explanations, including explaining data in relation to predictions and hypotheses.
Year 7
X
X
X
X
X
X
X
X
X
P rofessional
R esilient
O ptimistic
U nderstanding
D riven
Subject: Levers, moments and pressure
Science
Curriculum Coverage:
NC 2014 KS3: Motion and forces: Forces: moment as the turning effect of a force. work done and energy changes on deformation; Energy: Changes in systems: using physical processes and mechanisms, rather than energy, to explain the intermediate steps that bring about such changes; Energy: Energy changes and transfers: simple machines give bigger force but at the expense of smaller movement (and vice versa): product of force and displacement unchanged . Pressure in fluids: pressure measured by ratio of force over area – acting normal to any surface. Atmospheric pressure, decreases with increase of height as weight of air above decreases with height. Pressure in liquids, increasing with depth; upthrust effects, floating and sinking.
Lesson objective:
To describe the action of a lever identifying the key features and forces
Working Scientifically outcomes:
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety; Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective:
To describe the operation of a range of levers
Working Scientifically outcomes:
Use appropriate techniques, apparatus, and materials during fieldwork and laboratory work, paying attention to health and safety; Apply mathematical concepts and calculate results; Use and derive simple equations and carry out appropriate calculations.
Lesson objective:
To demonstrate the turning effect of a force and calculate the size of this effect
Working Scientifically outcome:
Use and derive simple equations and carry out appropriate calculations
Lesson objectives:
To verify the Principle of Moments and use it to find missing forces for objects in equilibrium
Working Scientifically outcomes:
Apply mathematical concepts and calculate results; Understand and use SI units and
IUPAC (International Union of Pure and Applied Chemistry) chemical nomenclature.
Lesson objective:
To apply the principle of conservation of energy to mechanical systems including levers and pulleys
Working Scientifically outcomes:
Present observations and data using appropriate methods, including tables and graphs;
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objective:
To explain the concept of pressure and scenarios where the pressure on a surface is increased or decreased
Working Scientifically outcomes:
Make predictions using scientific knowledge and understanding; Understand and use SI units and IUPAC (International Union of Pure and Applied Chemistry) chemical nomenclature.
Lesson objective:
To measure the pressure acting on a surface using the weight and the area of contact
Working Scientifically outcomes:
Apply mathematical concepts and calculate results; Understand and use SI units and
IUPAC (International Union of Pure and Applied Chemistry) chemical nomenclature.
Lesson objective:
To explain the cause of pressure in the atmosphere; to investigate the relationship between atmospheric pressure and height using secondary data
Working Scientifically outcomes:
Present observations and data using appropriate methods, including tables and graphs;
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Lesson objectives:
To examine the cause of pressure in liquids and describe it using Particle Theory
Working Scientifically outcome:
Interpret observations and data, including identifying patterns and using observations, measurements and data to draw conclusions.
Year 7
X
X
X
X
X
X
X
X
X
P rofessional
R esilient
O ptimistic
U nderstanding
D riven