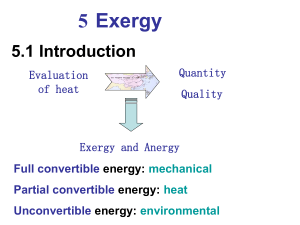
(exergy) cannot be negative.
advertisement

Thermodynamics First law of thermodynamics The first law of thermodynamics deals with the quantity of energy and asserts that energy cannot be created or destroyed. Second law of thermodynamics The second law deals with the quality of energy. Exergy E From book Fundamentals Energy is conserved in every device or process. It cannot be destroyed. Energy entering a system with fuel, electricity, flowing streams of matter, and so on can be accounted for in the products and by-products. However, the energy conservation idea alone is inadequate for depicting some important aspects of resource utilization. EXAMPLE Figure 7.1a shows an isolated system consisting initially of a small container of fuel surrounded by air in abundance. Suppose the fuel burns (Fig. 7.1b) so that finally there is a slightly warm mixture of combustion products and air as shown in Fig. 7.1c. The total quantity of energy associated with the system is constant because no energy transfers take place across the boundary of an isolated system. Still, the initial fuel–air combination is intrinsically more useful than the final warm mixture. For instance, the fuel might be used in some device to generate electricity or produce superheated steam, whereas the uses of the final slightly warm mixture are far more limited in scope. We can say that the system has a greater potential for use initially than it has finally. Since nothing but a final warm mixture is achieved in the process, this potential is largely wasted. More precisely, the initial potential is largely destroyed because of the irreversible nature of the process. Anticipating the main results of this chapter, exergy is the property that quantifies potential for use. The foregoing example illustrates that, unlike energy, exergy is not conserved but is destroyed by irreversibilities. Subsequent discussion shows that exergy not only can be destroyed by irreversibilities but also can be transferred to and from systems. Exergy transferred from a system to its surroundings without use typically represents a loss. Improved energy resource utilization can be realized by reducing exergy destruction within a system and/or reducing losses. An objective in exergy analysis is to identify sites where exergy destructions and losses occur and rank order them for significance. This allows attention to be centered on aspects of system operation that offer the greatest opportunities for cost-effective improvements. Environment and Dead State For thermodynamic analysis involving the exergy concept, it is necessary to model the atmosphere used in the foregoing discussion. The resulting model is called the exergy reference environment, or simply the environment. In this book the environment is regarded to be a simple compressible system that is large in extent and uniform in temperature, T0, and pressure, p0. In keeping with the idea that the environment represents a portion of the physical world, the values for both p0 and T0 used throughout a particular analysis are normally taken as typical ambient conditions, such as 1 atm and 258C (778F). Additionally, the intensive properties of the environment do not change significantly as a result of any process under consideration, and the environment is free of irreversibilities. When a system of interest is at T0 and p0 and at rest relative to the environment, we say the system is at the dead state. At the dead state there can be no interaction between system and environment, and thus no potential for developing work. Defining Exergy The discussion to this point of the current section can be summarized by the following definition of exergy: Exergy is the maximum theoretical work obtainable from an overall system consisting of a system and the environment as the system comes into equilibrium with the environment (passes to the dead state). Interactions between the system and the environment may involve auxiliary devices, such as the power cycle of Fig. 7.2, that at least in principle allow the realization of the work. The work developed is fully available for lifting a weight or, equivalently, as shaft work or electrical work. We might expect that the maximum theoretical work would be obtained when there are no irreversibilities. As considered in the next section, this is the case. TAKE NOTE... In this book, E and e are used for exergy and specific exergy, respectively, while E and e denote energy and specific energy, respectively. Such notation is in keeping with standard practice. The appropriate concept, exergy or energy, will be clear in context. Still, care is required to avoid mistaking the symbols for these concepts. Exergy of a System The exergy of a system, E, at a specified state is given by the expression where U, KE, PE, V, and S denote, respectively, internal energy, kinetic energy, potential energy, volume, and entropy of the system at the specified state. U0, V0, and S0 denote internal energy, volume, and entropy, respectively, of the system when at the dead state. In this chapter kinetic and potential energy are evaluated relative to the environment. Thus, when the system is at the dead state, it is at rest relative the environment and the values of its kinetic and potential energies are zero: KE0 5 PE0 5 0. By inspection of Eq. 7.1, the units of exergy are seen to be the same as those of energy. Equation 7.1 can be derived by applying energy and entropy balances to the overall system shown in Fig. 7.3 consisting of a closed system and an environment. See the box for the derivation of Eq. 7.1. Exergy Aspects In this section, we list five important aspects of the exergy concept: Exergy is a measure of the departure of the state of a system from that of the environment. It is therefore an attribute of the system and environment together. However, once the environment is specified, a value can be assigned to exergy in terms of property values for the system only, so exergy can be regarded as a property of the system. Exergy is an extensive property. The value of exergy cannot be negative. If a system were at any state other than the dead state, the system would be able to change its condition spontaneously toward the dead state; this tendency would cease when the dead state was reached. No work must be done to effect such a spontaneous change. Accordingly, any change in state of the system to the dead state can be accomplished with at least zero work being developed, and thus the maximum work (exergy) cannot be negative. Exergy is not conserved but is destroyed by irreversibilities. A limiting case is when exergy is completely destroyed, as would occur if a system were permitted to undergo a spontaneous change to the dead state with no provision to obtain work. The potential to develop work that existed originally would be completely wasted in such a spontaneous process. Exergy has been viewed thus far as the maximum theoretical work obtainable from an overall system of system plus environment as the system passes from a given state to the dead state. Alternatively, exergy can be regarded as the magnitude of the minimum theoretical work input required to bring the system from the dead state to the given state. Using energy and entropy balances as above, we can readily develop Eq. 7.1 from this viewpoint. This is left as an exercise. When a system is at the dead state, it is in thermal and mechanical equilibrium with the environment, and the value of exergy is zero. More precisely, the thermomechanical contribution to exergy is zero. This modifying term distinguishes the exergy concept of the present chapter from another contribution to exergy introduced in Sec. 13.6, where the contents of a system at the dead state are permitted to enter into chemical reaction with environmental components and in so doing develop additional work. This contribution to exergy is called chemical exergy. The chemical exergy concept is important in the second law analysis of many types of systems, in particular systems involving combustion. Still, as shown in this chapter, the thermomechanical exergy concept suffices for a wide range of thermodynamic evaluations. 7.4 Closed System Exergy Balance Like energy, exergy can be transferred across the boundary of a closed system. The change in exergy of a system during a process would not necessarily equal the net exergy transferred because exergy would be destroyed if irreversibilities were present within the system during the process. The concepts of exergy change, exergy transfer, and exergy destruction are related by the closed system exergy balance introduced in this section. The exergy balance concept is extended to control volumes in Sec. 7.5. Exergy balances are expressions of the second law of thermodynamics and provide the basis for exergy analysis. 7.6 Exergetic (Second Law) Efficiency The objective of this section is to show the use of the exergy concept in assessing the effectiveness of energy resource utilization. As part of the presentation, the exergetic efficiency concept is introduced and illustrated. Such efficiencies are also known as second law efficiencies Exergy (also called availability, work potential or available energy) is the maximum useful work that can be obtained from a system at a given state in a given environment; in other words, the most work you can get out of a system. In an exergy analysis the initial state is specified, the work output is maximized when the process between two specified states is executed in a reversible manner and the system must be in the dead state (meaning in thermodynamic equilibrium with the environment) at the end of the process to maximize the work output. In the last several decades, exergy analysis has begun to be used for system optimization. By analyzing the exergy destroyed by each component in a process, it should be made clear where to focus our efforts to improve system efficiency. It can also be used to compare components or systems to help make informed design decisions. Exergy associated with Kinetic and Potential Energy Kinetic and potential energy are forms of mechanical energy and can be converted to work entirely: 1 𝑒𝑘 + 𝑒𝑝 = 2 𝑣 2 + 𝑔𝑧 (kJ/kg). Reversible work Wrev Reversible work is the maximum useful work that can be obtained as a system undergoes a process between the specified initial and final states. This work output (or input) is obtained (or expended) when the process between the initial and final states is executed in a totally reversible manner. If the final state is the dead state, then it will be equal to the exergy. Irreversibility I Any difference between the reversible work 𝑊𝑟𝑒𝑣 and the useful work 𝑊𝑢𝑠𝑒𝑓𝑢𝑙 is due to the irreversibility present during the process, this difference is called irreversibility I. Irreversibility is the destroyed exergy (wasted work potential). It represents energy that could have been converted into work but was instead wasted. To have high system efficiency, we want 𝐼 to be as small as possible. Xdestroyed=I=ToSgen Second law efficiency 𝜼𝑰𝑰 The second law efficiency is defined as follows: 𝜂𝐼𝐼 = 𝑒𝑥𝑒𝑟𝑔𝑦 𝑟𝑒𝑐𝑜𝑣𝑒𝑟𝑒𝑑 𝑒𝑥𝑒𝑟𝑔𝑦 𝑑𝑒𝑠𝑡𝑟𝑜𝑦𝑒𝑑 =1− 𝑒𝑥𝑒𝑟𝑔𝑦 𝑠𝑢𝑝𝑝𝑙𝑖𝑒𝑑 𝑒𝑥𝑒𝑟𝑔𝑦 𝑠𝑢𝑝𝑝𝑙𝑖𝑒𝑑 thermal efficiency and the reversible (maximum) thermal efficiency. 𝜂𝐼𝐼 = 𝜂𝑡ℎ 𝜂𝑡ℎ,𝑟𝑒𝑣 = 𝑊𝑢𝑠𝑒𝑓𝑢𝑙 𝑊𝑟𝑒𝑣 We can calculate the exergy, X (work potential) at a given state. The work potential is a function of the total energy of the system. X X KE X PE X internal energy X flow work (remember that in a control mass, there will be no flow work) XKE (exergy due to kinetic energy): V2/2 (on a per unit mass basis XPE: gZ Xinternal energy: u-uo+Po(v-vo)-To(s-s0) Exergy of fixed mass X = (U-U0)+P0(V – V0)–T0(S – S0) +½mVel2+mgz Φ = (u-u0)+P0(v-v0)-T0(s-s0)+½Vel2+gz or Φ = (e-e0)+P0(v-v0)-T0(s-s0) Note that Φ = 0 at dead state If the state of system or the state of the environment do not change, the exergy does not change Exergy change of steady flow devices, nozzles, compressors, turbines, pumps, heat exchangers; is zero during steady operation. Exergy of a closed system is either positive or zero. Exergy of a flow stream Flow exergy Ψ=(h-h0)-T0(s-s0)+½Vel2+gz Exergy transfer by heat work and mass Like energy, exergy can be transferred in three forms: Heat o o Work o o o Mass o o Xheat =(1-T0/T)Q When T not constant, then Xheat =∫(1-T0/T)δQ Xwork = W – Wsurr (for boundary work) Xwork = W (for all other forms of work) Where Wwork = P0(V2-V1) Mass contains exergy as well as energy and entropy X=m Ψ=m[(h-h0)-T0(s-s0)+½Vel2+gz]