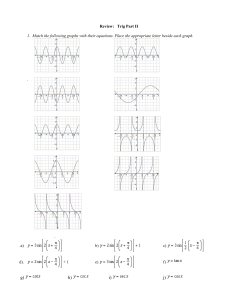
chemquiz_dec1
advertisement

SNC 1P - Chemistry Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true. ____ 1. A neutron is a positively charged particle in an atom. ____________________ ____ 2. A proton is a particle in an atom that has no electrical charge. ____________________ ____ 3. Protons and electrons are found in the nucleus of an atom. ____________________ ____ 4. Most elements are non-metals. ____________________ ____ 5. A material that can be pulled into a long, thin wire is malleable. ____________________ ____ 6. Conductivity is a material’s ability to conduct thermal energy or electricity. ____________________ ____ 7. A molecule is a group of two or more atoms that are chemically joined together. ____________________ ____ 8. A molecule of oxygen is an element. ____________________ ____ 9. If there is only one symbol in a chemical formula, the substance is a(n) element. ____________________ ____ 10. An element is a simpler substance than a compound. ____________________ ____ 11. The molecule H2SO4 contains six atoms. ____________________ ____ 12. To create a model of aspirin (C9H8O4), you need twenty atoms in total. ____________________ Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 13. Which of the following is an example of a chemical property? a. state of matter b. brittleness c. flammability d. Odour ____ 14. Which of the following characteristics is a chemical property of oxygen? a. colourless b. odourless c. readily supports combustion d. slightly soluble in water ____ 15. Which of the following is an example of a chemical change? a. crumpling a piece of paper b. melting ice c. baking a cake d. deflating a basketball ____ 16. Which of the following is an example of a physical change? a. salt dissolving in water b. fireworks exploding c. hair colouring d. iron rusting ____ 17. What is the name given to the temperature at which a substance changes from a liquid to a solid? a. melting point b. freezing point c. boiling point d. Density ____ 18. Which of the following gases burns so quickly in air that it often produces a loud “pop” when it is ignited? a. oxygen b. hydrogen c. carbon dioxide d. Nitrogen ____ 19. Which of the following particles are found in the nucleus of an atom? a. protons and electrons b. protons and neutrons c. electrons and neutrons d. electrons and orbits ____ 20. Which of the following is a negatively charged particle in an atom? a. proton b. neutron c. electron d. Nucleus ____ 21. How many electrons does this atom contain? a. b. c. d. 3 4 6 10 ____ 22. Each element’s box in the periodic table contains which of the following? a. chemical symbol b. element’s name c. atomic number d. all of the above ____ 23. Which non-metal makes up 78 % of the air we breathe? a. nitrogen b. oxygen c. argon d. Helium ____ 24. Which atomic model shows an atom of nitrogen? a. b. c. d. ____ 25. Which term describes the small number that follows the symbol for an element in a chemical formula and shows how many atoms of the element are present? a. molecule b. subscript c. electrolysis d. Catalyst ____ 26. Water is an example of which of the following? a. element b. compound c. mixture d. Solution ____ 27. What is the chemical formula for water? a. HO2 b. H2O c. H3O d. H2O2 ____ 28. Which of the following represents an element? a. Cl2 b. MgCl2 c. NO d. KCl ____ 29. Which term describes a chemical change involving oxygen and a fuel that releases large amounts of energy? a. rusting b. burning c. decomposition d. Electrolysis ____ 30. How many atoms does a molecule of calcium carbonate (CaCO3) contain? a. 3 b. 4 c. 5 d. 6 SNC 1P - Chemistry Answer Section MODIFIED TRUE/FALSE 1. ANS: F, proton PTS: 1 LOC: C3.1 2. ANS: F, neutron REF: K/U OBJ: 6.1 Atoms and Elements MSC: What Do You Remember? PTS: 1 LOC: C3.1 3. ANS: F, neutrons REF: K/U OBJ: 6.1 Atoms and Elements MSC: What Do You Remember? PTS: 1 LOC: C3.1 4. ANS: F, metals REF: K/U OBJ: 6.1 Atoms and Elements MSC: What Do You Remember? PTS: 1 REF: K/U MSC: What Do You Remember? 5. ANS: F, ductile 6. 7. 8. 9. 10. 11. PTS: MSC: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: 1 REF: K/U What Do You Remember? T 6.3 Metals LOC: C2.1 T 7.2 Putting Atoms Together T 7.2 Putting Atoms Together T 7.2 Putting Atoms Together T 7.2 Putting Atoms Together F, seven OBJ: 6.3 Metals LOC: C3.3 OBJ: 6.3 Metals LOC: C2.1 PTS: MSC: PTS: LOC: PTS: LOC: PTS: LOC: PTS: LOC: 1 REF: K/U What Do You Remember? 1 REF: K/U C2.1 MSC: What Do You Remember? 1 REF: K/U C3.2 MSC: What Do You Understand? 1 REF: K/U C3.6 MSC: What Do You Understand? 1 REF: A C3.2 MSC: What Do You Understand? PTS: 1 REF: T/I OBJ: 7.2 Putting Atoms Together LOC: C3.6 MSC: What Do You Understand? 12. ANS: F, twenty-one PTS: 1 LOC: C2.6 MULTIPLE CHOICE REF: A OBJ: 7.3 Matching Models and Formulas MSC: What Do You Understand? 13. ANS: OBJ: MSC: 14. ANS: OBJ: MSC: 15. ANS: LOC: 16. ANS: LOC: 17. ANS: LOC: 18. ANS: LOC: 19. ANS: LOC: 20. ANS: LOC: 21. ANS: LOC: 22. ANS: LOC: 23. ANS: LOC: 24. ANS: LOC: 25. ANS: LOC: 26. ANS: LOC: 27. ANS: LOC: 28. ANS: LOC: 29. ANS: OBJ: MSC: 30. ANS: LOC: C PTS: 1 REF: K/U 5.3 Chemical Properties: How Substances Change What Do You Understand? C PTS: 1 REF: A 5.3 Chemical Properties: How Substances Change What Do You Understand? C PTS: 1 REF: A C2.1 MSC: What Do You Understand? A PTS: 1 REF: A C2.1 MSC: What Do You Understand? B PTS: 1 REF: K/U C2.1 MSC: What Do You Remember? B PTS: 1 REF: T/I C2.7 MSC: What Do You Remember? B PTS: 1 REF: K/U C3.1 MSC: What Do You Remember? C PTS: 1 REF: K/U C3.1 MSC: What Do You Remember? A PTS: 1 REF: T/I C3.1 MSC: Solve a Problem D PTS: 1 REF: K/U C3.3 MSC: What Do You Remember? A PTS: 1 REF: A C3.4 MSC: Solve a Problem C PTS: 1 REF: T/I C3.1 MSC: Create and Evaluate B PTS: 1 REF: K/U C2.1 MSC: What Do You Remember? B PTS: 1 REF: A C3.2 MSC: What Do You Understand? B PTS: 1 REF: C C3.6 MSC: What Do You Remember? A PTS: 1 REF: T/I C3.2 MSC: Solve a Problem B PTS: 1 REF: K/U 7.8 Oxygen: Properties, Uses, and Impacts What Do You Remember? C PTS: 1 REF: T/I C3.6 MSC: Solve a Problem LOC: C2.1 LOC: C1.1 OBJ: 5.4 Physical and Chemical Changes OBJ: 5.4 Physical and Chemical Changes OBJ: 5.7 Unique Properties OBJ: 5.10 What's This Gas? OBJ: 6.1 Atoms and Elements OBJ: 6.1 Atoms and Elements OBJ: 6.1 Atoms and Elements OBJ: 6.1 Atoms and Elements OBJ: 6.4 Non-metals OBJ: 6.1 Atoms and Elements OBJ: 7.2 Putting Atoms Together OBJ: 7.2 Putting Atoms Together OBJ: 7.2 Putting Atoms Together OBJ: 7.2 Putting Atoms Together LOC: C2.1 OBJ: 7.2 Putting Atoms Together