File - Pacific Honors Program Study Site!
advertisement

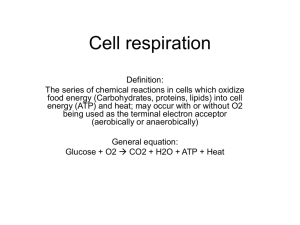
Chapter Nine: Cellular Respiration We eat because we need: energy and carbon for building polymers of our own. -Autotrophs get their carbon from CO₂, however heterotrophs eat other organisms for carbon. Organic molecules (molecules that contain carbon) contain energy. Cells use enzymes to break down organic molecules for energy. Catabolic pathways break down the organic molecules for energy. Some energy is released as heat and some is used to do work. One catabolic process is called fermentation is a partial breakdown of sugars. This occurs WITHOUT oxygen. The more effective pathway is cellular respiration, where oxygen is used as reactant along with organic molecules. Mitochondria do most of cellular respiration. So this is the overview of cellular respiration: Organic compounds + oxygen = carbon dioxide + water + energy Carbohydrates, fats, and proteins can all be used as fuel. In cellular respiration, we look at the breaking down of glucose. ∆G= -686 kcal/mol The breakdown of glucose is exergonic, meaning it releases energy. The specific amount of energy released is -686 kcal. Cellular respiration gets energy through decomposing glucose because of the transfer of electrons during the chemical reactions. The transfer of electrons releases energy in molecules, and this energy is used to create ATP. In many chemical reactions, there is a transfer of one or more electrons (e⁻) from one reactant to another. These electron transfers are called oxidation-reduction reactions, or redox reactions for short. In a redox reaction, the loss of an electron from a reactant is called oxidation. The addition of an electron to a reactant is called reduction. The electron donor is called the reducing agent. The electron receiver is called the reducing agent. Sometimes redox reactions do not involve the complete transfer of electrons. Electronegativity can also be part of a redox reaction In the beginning of this reaction, methane is a nonpolar. The sharing of electrons between the carbon and hydrogen are equal, because the electronegativity of hydrogen and carbon and the same. However, after the reaction, carbon dioxide is formed and now the electrons are shared unequally because oxygen is more electronegative than hydrogen. So Methane has been oxidized. The oxygen molecule has been reduced When this sort of thing happens (when an electron shifts from a less electronegative atom to a more electronegative one), potential energy is released. So energy is released when this happens! So organic molecules with lots of hydrogens are great fuel, because they have lots of electrons to go through these types of reactions. Glucose and other organic fuels are broken apart during steps of cellular respiration. At these steps, hydrogens are taken away from glucose, and are taken up by an enzyme called NAD+. NAD+ is an oxidizing reaction. NAD+ picks up electrons because enzymes called dehydrogenases remove a pair of hydrogen atoms from a glucose. It removes two electrons and two protons. The reduced form of NAD+ is NADH. NADH store energy in electrons until it can be used to make ATP. One step of respiration is the electron transport chain. This is a series of reactions in which NAD+ donates electrons to the electron transport chain. Electrons are passed down in a series of steps and finally they “fall” to the most electronegative step of the electron transport chain, which is oxygen. The electron transport chain is built into the inner membrane of mitochondria. At the last step of the chain, electrons bind with oxygen, creating water. THE PROCESS OF CELLULAR RESPIRATION Respiration has three stages! 1. Glycolysis 2. The Krebs Cycle 3. The electron transport change and oxidative phosphorylation Both glycolysis and the Krebs Cycle are the catabolic pathways that break down glucose and other fuels. Glycolysis occurs in the cytosol and breaks glycolysis into two molecules of pyruvate. Krebs Cycle occurs in the mitochondrial matrix (the inside of mitochondria) and degrades pyruvate to carbon dioxide. Some of the steps in these stages involve redox reactions, so lots of NADH is made. In the electron transport chain, electrons from NADH are passed down from molecule to molecule, providing energy at each step. This energy is used in oxidative phosphorylation, which is the synthesis of ATP using energy from redox reactions of the electron transport chain. Most of the ATP produced by cellular respiration is produced by oxidative phosphorylation. However, some is created during glycolysis and Krebs cycle by substrate-level phosphorylation. This is when and enzyme transfers a phosphate group to ADP. Each molecule of glucose = 38 ATP molecules A Closer Look at Glycolysis In this stage, a molecule of glucose is split in half to form two molecules of pyruvate. Important enzymes: phosphofructokinase. There are two phases of glycolysis: The energy investment phase and the energy pay-off phase. The energy investment phase is when the cell actually uses 2 ATP to get things moving. The energy payoff phase is when four ATP are formed from substrate level phosphorylation. Also, 2 NADH molecules are reduced. So after glycolysis we have a net energy yield of: 2 ATP 2 pyruvate and 2 NADH In: glucose and 2 ATP. Out: 2 3-carbon molecules of pyruvate, 4 ATP and 2 NADH. NOTE THAT GLYCOLYSIS REQUIRED NO OXYGEN. It is anaerobic, since it required NO oxygen to take place. A Closer Look at the Krebs Cycle Glycolysis barely released any energy. So now most of the energy is still in the pyruvate molecules. So now if oxygen is present, then the pyruvate molecules will enter a mitochondrion. Then the pyruvates are turned into a molecule called acetyl CoA. This is a step between glycolysis and Krebs cycle. This step has three parts. It takes place in the mitochondrial matrix. 1. Pyruvate’s carboxyl group is removed and given off as carbon dioxide. 2. The two-carboned molecule left over is oxidized to form a molecule called acetate. And one NADH is oxidized per pyruvate. 3. Finally, coenzyme A is attached to the acetate. Ok so now acetyl CoA can go through Krebs Cycle. There are eight steps. Each step is catalyzed by a specific enzyme. In the Krebs Cycle, there is another oxidizing agent (electron acceptor like NAD+) and it’s called FAD+. Its reduced form is FADH₂ The products of the Krebs Cycle include 8 NADH, 2 FADH2, 6 CO₂ and 2 ATP by substrate level phosphorylation. Note: The Krebs Cycle is AEROBIC, because it requires oxygen!! The Electron Transport Chain and ATP synthesis This step makes most of the ATP. In: 10 NADH and 2 FADH₂ Out: 10 NAD+ and 10 H+ and 2 FAD At this point, molecules of FADH₂ and NADH release their electrons at the electron transport chain. The energy the results from the electrons going down the steps of the electron transport chain is used to power oxidative phosphorylation. So electron transport chains are embedded in the inner membrane. Cristae allow there to be lots of room for electron transport chains. Most of the molecules of the electron transport chain are proteins. These proteins have another molecule bound to them called a “prosthetic group”. These groups accept and lose electrons down the chain. The first protein is called a flavoprotein. The second protein is an iron and sulfur protein. The third molecule in the electron chain is called ubiquinone, and it is a lipid, not a protein. The rest of the electron carriers in the chain are proteins called cytochromes. Their prosthetic group is a heme group, made of four organic rings and a single iron atom. Finally, electrons “fall” down to oxygen (O₂) and water is the product. So all this energy made from the reactions along the electron transport chain is used to make ATP. Chemiosmosis In the inner membrane of the mitochondrion are many copies of ATP synthase, which is an enzyme that makes ATP. ATP synthase uses an ion gradient to make ATP. The ion gradient is hydrogen ions inside and outside a cell. Energy from the electron transport chain pumps H+ across the membrane, from the mitochondrial matrix into the intermembrane space. H+ molecules are just released by the electron transport chain often. When H+ diffuses back into the matrix, it passes through ATP synthase, and ATP harnesses this flow and creates ATP. The H+ gradient couples the energy from the redox reactions in the electron transport chain to ATP synthesis. This process is called chemiosmosis. The H+ gradient is called a proton-motive force. ATP synthase has three parts: A cylinder in the inner mitochondrial membrane, a knob that is inside the mitochondrial matrix, and a internal rod connecting the two. When H+ ions flow through ATP synthase, they cause the cylinder and rod to rotate, which causes the knob to make ATP. So to sum it up: Chemiosmosis is an energy-coupling mechanism that uses energy stored in the form of an H+ gradient to drive cellular work. In respiration, most energy is created through Glucose—NADH—electron transport chain— proton-motive force—ATP. One molecule of NADH can provide enough energy to make 3 ATP. FADH₂ has enough energy to make 2 ATP. Respiration is 40% effective, the rest of the energy is lost as heat. Other Metabolic processes Sometimes, organisms live in environments without oxygen, and they need to have some kind of cell respiration that doesn’t require oxygen. Environments with oxygen are called aerobic, environments without oxygen are called anaerobic. Organisms that do not use oxygen during their respiration use fermentation. There are many different types of fermentation. Mostly fermentation is just glycolysis with a few other parts. Sometimes NAD+ will edit the ending product In alcohol fermentation, pyruvate from glycolysis is converted to ethanol (alcohol) In lactic acid fermentation, pyruvate is reduced to form lactate. When you are running, your muscle called start making ATP by lactic acid fermentation because oxygen is scarce. When your muscles are sore, its because of a buildup of lactic acid. So, respiration is much more effective than fermentation because respiration has the electron transport chain. Some organisms can switch back and forth back and forth between respiration and fermentation. For example, our muscle cells switch between the two. These organisms are called facultative anaerobes. A cell can control its metabolism. If there is a lot of ATP, the cell will slow respiration. One molecule that is an off switch for metabolism is phosphofructokinase (an enzyme of glycolysis). This enzyme starts respiration, so if it is controlled, then respiration is controlled. Phosphofructokinase is inhibited by ATP and stimulated by AMP. This is feedback regulation. DONE!