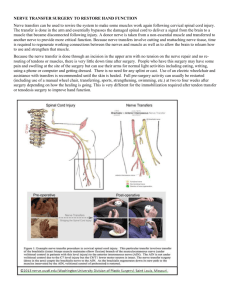
Consultation Protocol (Word 127 KB)
advertisement

1263 Consultation Decision Analytic Protocol (DAP) to guide the assessment of intra-abdominal vagal nerve modulation for the management of obesity October 2012 Table of Contents MSAC and PASC ....................................................................................................................... 2 Purpose of this document ........................................................................................................... 2 Purpose of application ............................................................................................................. 4 Background.............................................................................................................................. 4 Current arrangements for public reimbursement .......................................................................... 4 Regulatory status ....................................................................................................................... 4 Intervention ............................................................................................................................ 6 Description ................................................................................................................................ 6 Delivery of the intervention ........................................................................................................ 7 Prerequisites ............................................................................................................................. 8 Co-administered and associated interventions ............................................................................. 8 Listing proposed and options for MSAC consideration .......................................................... 10 Proposed MBS listing .................................................................................................................10 Clinical place for proposed intervention ......................................................................................17 Comparator ............................................................................................................................ 20 Clinical claim .......................................................................................................................... 20 Outcomes and health care resources affected by introduction of proposed intervention.............................................................................................................. 22 Clinical outcomes ......................................................................................................................22 Health care resources ...............................................................................................................23 Proposed structure of economic evaluation (decision-analytic) ........................................... 25 Attachment A ......................................................................................................................... 28 MBS items relating to the placement and removal of neurostimulators or pulse generators and associated leads and electrodes....................................................................................28 MSAC and PASC The Medical Services Advisory Committee (MSAC) is an independent expert committee appointed by the Minister for Health and Ageing (the Minister) to strengthen the role of evidence in health financing decisions in Australia. MSAC advises the Minister on the evidence relating to the safety, effectiveness, and cost-effectiveness of new and existing medical technologies and procedures and under what circumstances public funding should be supported. The Protocol Advisory Sub-Committee (PASC) is a standing sub-committee of MSAC. Its primary objective is the determination of protocols to guide clinical and economic assessments of medical interventions proposed for public funding. Purpose of this document This document is intended to provide a draft decision analytic protocol that will be used to guide the assessment of an intervention for a particular population of patients. The draft protocol will be finalised after inviting relevant stakeholders to provide input to the protocol. The final protocol will provide the basis for the assessment of the intervention. The protocol guiding the assessment of the health intervention has been developed using the widely accepted “PICO” approach. The PICO approach involves a clear articulation of the following aspects of the question for public funding the assessment is intended to answer: Patients – specification of the characteristics of the patients in whom the intervention is to be considered for use Intervention – specification of the proposed intervention and how it is delivered Comparator – specification of the therapy most likely to be replaced by the proposed intervention Outcomes – specification of the health outcomes and the healthcare resources likely to be affected by the introduction of the proposed intervention Summary of matters for which PASC seeks input The PASC invites comment on the following matters: • description of the typical management of a patient undergoing intra-abdominal vagal nerve modulation therapy compared with a patient having undergone other types of bariatric surgery such as gastric banding; o details of the typical schedules for blood tests, imaging, consultations with allied health professionals such as dieticians, exercise physiologists, psychologists, routine monitoring/adjustments by physician, routine replacement of the device, and monitoring and management of adverse events • whether services related to the implant, adjustment and removal of intra-abdominal vagal nerve modulation therapy device is sufficiently similar, in terms of technical aspects, to placement, adjustment and removal services reimbursed under the MBS for other neuroregulators (especially those used in the treatment of neuropathic pain) and thus, whether fees for these other services can serve as a guide as to an appropriate fee for the requested services • whether there are any implications of a large differential between the MBS Schedule fee and the fee likely to be charged in the market for services related to intra-abdominal vagal nerve therapy that need to be considered and addressed • whether an MBS listing should be limited to adult patients with clinically severe obesity • whether a patient group exists in whom other bariatric surgeries are unsuitable but where intra-abdominal vagal nerve modulation therapy would be suitable • that women experiencing difficulties with fertility due to obesity would be adequately covered by a listing limiting use to adult patients with clinically severe obesity is valid • whether there is a clinical place for intra-abdominal vagal nerve modulation therapy and for other forms of bariatric surgery intervention in the super obese who need to lose some weight prior to gastric bypass • whether intra-abdominal vagal nerve modulation therapy will compete with other forms of bariatric surgery (particularly gastric banding) in patients with clinically severe obesity • the range of outcomes upon which comparative effectiveness and safety should be determined • the issue of the extent to which changes in weight are an acceptable surrogate for longer term outcomes such as the prevalence of co-morbidities and, as a consequence, survival. Purpose of application A proposal for an application requesting MBS listing of surgical procedures for implanting and removing an implantable medical device that modulates the activity of the vagal nerve, which is used in the management of obesity, was received from Device Technologies Australia Pty Ltd by the Department of Health and Ageing in March 2012. The proposal relates to an intervention that is not currently reimbursed under the MBS. The Deakin Health Economics Unit at Deakin University, under its contract with the Department of Health and Ageing, has drafted this decision analytical protocol to guide the preparation of an assessment of the safety, effectiveness and cost-effectiveness of intra-abdominal vagal nerve modulation when used in the management of obesity to inform MSAC’s decision-making regarding public funding of the intervention. Background Current arrangements for public reimbursement Intra-abdominal vagal nerve modulation therapy is currently not publicly funded. Regulatory status Current approvals by the Australian Register of Therapeutic Goods (ARTG) for devices that are used in intra-abdominal vagal nerve modulation therapy are summarised in Table 1. Table 1: Australian Register of Therapeutic Goods listings of devices used in intra-abdominal vagal nerve modulation therapy ARTG Class* GMDN Name Number Unique Product Identifier Intended purpose The neuroregulator and leads are intended to be used as part of the Maestro Rechargeable System to generate and deliver vagal 192882 blocking (VBLOC) therapy to the vagal nerve for weight reduction in obese patients. The subcutaneously implanted rechargeable neuroregulator is EnteroMedics Gastric contractility intended to be used as part of the Maestro Rechargeable System Maestro 192883 AIMD modulation system to generate vagal blocking (VBLOC) therapy for weight reduction in Rechargeable pulse generator obese patients. Neuroregulator The implanted lead is intended to be used as part of the Maestro Electrode/lead, EnteroMedics Rechargeable System to deliver vagal blocking (VBLOC) therapy to stimulator, Maestro Anterior the anterior trunk of the vagal nerve for weight reduction in obese 193393 III implantable, vagus Lead patients. nerve The implanted lead is intended to be used as part of the Maestro Electrode/lead, EnteroMedics Rechargeable System to deliver vagal blocking (VBLOC) therapy to stimulator, 193394 III Maestro Posterior the posterior trunk of the vagal nerve for weight reduction in obese implantable, vagus Lead patients nerve The external device is intended to be used as part of the Maestro Active-implantable- EnteroMedics Rechargeable System to provide the power necessary to recharge 193373 III device Maestro Mobile the neuroregulator battery and provide a communications path communicator Charger between the clinician programmer and neuroregulator. The external transmit coil is intended to be used as part of the Active-implantable- EnteroMedics Maestro Rechargeable System during recharging and to provide a Maestro Patient communications path between the clinician programmer and 194020 III device communicator Transmit Coil neuroregulator. The external transmit coil is intended to be used as part of the Active-implantable- EnteroMedics Maestro Rechargeable System during recharging and to provide a 194021 III device Maestro Patient communications path between the clinician programmer and communicator Transmit Coil neuroregulator. The external transmit coil is intended to be used in the operating Active-implantable- EnteroMedics room as part of the Maestro Rechargeable System to provide a 193395 III device Maestro Clinician communications path between the clinician programmer and communicator Transmit Coil neuroregulator. The programmer and cable are intended to be used as part of the Programmer, EnteroMedics Maestro Rechargeable System to configure, monitor and change implantable 193392 III Maestro Clinician settings of the implanted neuroregulator. stimulator, vagus Programmer Kit nerve The external devices are intended to be used as part of the Active-implantable- EnteroMedics Maestro Rechargeable System to provide the power necessary to 193391 III device Maestro Patient recharge the neuroregulator battery and provide a communications communicator Kit path between the clinician programmer and neuroregulator. Cable to connect a Clinical Programmer (laptop) with the Mobile 193038 I Cable, <specify> N/A Charger. A device which connects to a power outlet and an AC recharger port on a mobile charger in order to charge a battery, restoring the 193040 I Battery charger N/A battery to an appropriate working condition A belt used to stabilize the position of a transmit coil during the 195224 I Holder, <specify> N/A recharging or communication with the implanted device. ABBREVIATIONS: ARTG – Australian Register of Therapeutic Goods; GMDN – Global Medical Device Nomenclature; AIMD – active implantable medical device * Medical devices are classified in to 5 classes based on the level of risk and the intended purpose of the device: I – low risk; IIa – low-medium risk; IIb – medium-high risk; III – high risk; AIMD – high risk active implantable medical device Gastric contractility EnteroMedics AIMD modulation system Maestro Implant Kit Intervention Description Currently only one system is registered by the TGA for intra-abdominal vagal nerve modulation therapy - the Maestro Rechargeable System™ (the device). This specific system is described as consisting of a neuroregulator and two implantable flexible leads with electrodes. The electrodes are placed laparoscopically, under general anaesthesia, at the anterior and posterior intra-abdominal vagal nerve trunks and connected to the implantable neuroregulator, which is placed subcutaneously on the abdominal wall below the rib margin. The neuroregulator is powered by an internal rechargeable battery. A mobile, external controller that is connected via a small, flexible cable to a cutaneous transmit coil positioned over the implanted device allows the physician to control and upload information from the device. Treatment can be reversed by a physician turning off the neuroregulator. MBS listing of the following three surgical procedures is proposed: (i) subcutaneous placement of an implantable neuroregulator; (ii) placement of associated leads and electrodes so that they are connected to the vagal nerve; and (iii) removal of the neuroregulator and electrodes. The neuroregulator and associated devices are used to modulate the activity of the vagal nerve. Billington et al, 20091, report that intermittent blocking of the activity of the vagal nerve by means of a neuroregulator may lead to weight loss via several potential mechanisms including: inhibition of gastric accommodation leading to early satiation (fullness); and, inhibition of gastric contractions leading to enhanced satiety (reduced hunger). Intra-abdominal vagal nerve modulation therapy has been investigated and is proposed for use in the management of obesity. For the review of Medical Benefit Schedule (MBS) items for the surgical treatment of obesity (Application 1180r) considered by MSAC in November 2011, obesity was generally defined as a disease in which fat has accumulated to the point where health is impaired, and more specifically, defined as a body mass index (BMI) of over 30 kg/m2 for adults. BMI is an index of weight-for-height that is commonly used to classify overweight and obesity in adults. BMI is calculated as weight in kilograms divided by the square of height in metres. “Clinically severe obesity” is defined as BMI ≥ 40 kg/m2, or between 35 kg/m2 and 40 kg/m2 where there are other major medical conditions such as high blood pressure or diabetes. As discussed in further detail in the section titled “Proposed MBS listing”, which starts on p.10 below, PASC considered the primary target population for intraabdominal vagal nerve modulation therapy would be patients with clinically severe obesity. Obesity is a multifactorial disease that may have a variety of underlying causes, including physical illness, genetics, behavioural and psychological factors, and lifestyle choices. Obesity is associated 1 Billington C.J., Kow L., Collins J., Wray N.H., Tweden K.S., Vollmer M.C., Wilson R.R., Yurik T.M., Freston J.W. and Toouli J. Correlations between enhanced satiety and reduced calorie intake during intermittent vagal block (VBLOC Therapy) to treat obesity. Gastroenterology 2009;136:5 SUPPL. 1 (A386) with an increased risk for a number of comorbidities including diabetes, cardiovascular disease, high blood pressure, stroke, high cholesterol, obstructive sleep apnoea, osteoarthritis, and some cancers 2,3 Delivery of the intervention During a laparoscopic surgical procedure, the surgeon makes three to five incisions to implant the electrodes. Through the incisions, the surgeon places small electrodes around both of the patient's vagal nerve trunks near the bottom of the oesophagus. The physician then places the neuroregulator under the skin. The device connectivity is checked by an impedance assessment. The patient requires an overnight stay in hospital due to the use of a general anaesthetic in the vast majority of cases. The requirement for an overnight hospital stay is driven by the need for observation as the reaction general anaesthesia is unpredictable, to monitor the patient’s reaction to the implanted device and due to patient potentially being at high risk of complications due to co-morbidities. Patients are given information about intra-abdominal vagal nerve modulation therapy, and its care including programming, interrogation and the need to charge the device each day, etc. Once implanted, the device remains in the patient. Treatment can be started and stopped by a physician turning on/off the neuroregulator. The intensity of the signal can be strengthened or weakened to suit the individual patient. The signal is programmed (by the physician) to be on for twelve plus hours per day and programmed to be turned off during the night (as there is usually no need to have appetite suppressed during the night). The implanted part of the device consists of leads with electrodes and a neuroregulator. The initial follow-up of a patient following placement of the device involves: a consultation for the programming of the device and initiation of therapy in the 2 nd week following placement a consultation for adjustment of the amplitude of the signal delivered by the device (according to patient tolerance) in the 4th week following placement. Further adjustments, if necessary, could be made by nurse practitioners (under supervision of a physician). Beyond the initial follow up, it is claimed that follow-up is comparable to follow-up given to patients undergoing other forms of bariatric surgery-in that regular medical visits would be used to monitor a patient’s weight loss and to monitor for adverse events. The applicant anticipates that the utilisation of ancillary services e.g., blood tests, imaging, consultations with allied health professionals such as dieticians, exercise physiologists and psychologists, would be being similar as in the gastric banding setting. The sponsor indicates that the neurostimulator is likely to be required to be replaced approximately every 5 years. However, leads and electrodes are anticipated to require replacing approximately every 15-20 years. Each of these would require a surgical procedure. 2 3 Source: Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. Oct 13 2004;292(14):1724-1737. Source: Australian Bureau of Statistics, 2012. Overweight and Obesity in Adults, Australia, 2004-5. Catalogue number 4719.0 - Available online at: http://www.abs.gov.au/ausstats/abs@.nsf/mf/4719.0/ Prerequisites The proposal describes that the procedure would be delivered by bariatric surgeons who are members of the Obesity Surgery Society of Australia and New Zealand (OSSANZ). OSSANZ would determine the training and accreditation requirements. It is proposed that the medical service should be funded only if delivered directly by a bariatric surgeon. PASC noted that a review of existing MBS items for bariatric surgery (Application 1180r) that was considered by MSAC in November 2011 reported that there is a steep learning curve associated with bariatric surgery. Best practice guidelines relating to weight loss surgery recommend that bariatric surgeons undertake the same procedures frequently (50-100 cases per year) operating in properly equipped, high volume weight loss centres with integrated and multidisciplinary treatment 4. PASC advised that any application to MSAC should include a description of the strategies that would be implemented to ensure safety and best practice surgery particularly if the likely number of patients that will undergo intra-abdominal vagal nerve modulation therapy will be low (e.g., 100-200 per year as predicted by the proposal for an application [Part B - eligibility). The projections of use in the proposal appear to be low, especially when considered alongside utilisation of MBS items used for other bariatric surgeries as shown in Table 2. It should be noted that some items included in this table (e.g., MBS item 30518 - partial gastrectomy), while including procedures for management of obesity (e.g., sleeve gastrectomy), will also include use of the procedure for other indications. The proposal suggests that the number of patients using intraabdominal vagal nerve modulation therapy will be limited by the number of surgeons trained to perform the procedure and by the positioning of the intervention relative to other forms of bariatric surgery. Table 2: Utilisation of MBS items relating to bariatric surgery for obesity MBS Item MBS item descriptor number 30511 MORBID OBESITY, gastric reduction or gastroplasty for, by any method 30512 MORBID OBESITY, gastric bypass for, by any method including anastomosis 30518 PARTIAL GASTRECTOMY Utilisation in 2011 9,401 354 1,969 Co-administered and associated interventions The surgical procedures for implanting and removing the implantable medical device used to deliver intra-abdominal vagal nerve modulation therapy for which listing on the MBS is to be requested, will also involve the services of an anaesthetist and an assistant surgeon. In addition, patients will require supply of the devices required to deliver intra-abdominal vagal nerve modulation therapy (e.g., the neuroregulator and implantation kit and other items listed in Table 1). 4 Source: Kelly J, Tarnoff M, Shikora S, Thayer B, Jones DB, Forse RA et al 2005, ‘Best practice recommendations for surgical care in weight loss surgery’, Obesity Research, 13: 227- 233. The proposal states that the device is currently priced at $17,850. This cost represents the device portion of intra-abdominal vagal nerve modulation therapy. Currently the device is not listed on the Prostheses List and listing would be conditional on the device meeting the five criteria for listing on the Prostheses List, including the criterion that the professional service associated with the implantation of the device must have a relevant MBS item number. In considering the Review of items for the surgical treatment of obesity in November 2011, MSAC agreed that bariatric surgery interventions should be performed in the context of a multidisciplinary service – with a concentration of surgical experts working not only with physicians, but also with dieticians, exercise physiologists, and psychologists. As discussed in the section titled “Delivery of the intervention” above, it is anticipated that follow-up following placement of the device will comparable to follow-up given to patients undergoing other forms of bariatric surgery. PASC considered that regular medical visits would be used to monitor a patient’s weight loss and to monitor for adverse events. The applicant anticipates that the utilisation of ancillary services e.g., blood tests, imaging, consultations with allied health professionals such as dieticians, exercise physiologists and psychologists, would be being similar as in the gastric banding setting. Listing proposed and options for MSAC consideration Proposed MBS listing The items proposed by the applicant for inclusion on the MBS relevant to intra-abdominal vagal nerve modulation therapy are summarised in Table 3. Table 3: Proposed MBS item descriptors Category 3 - THERAPEUTIC PROCEDURES MBS Item number XXXX NEUROREGULATOR, subcutaneous placement of, including placement and connection of electrodes, to generate vagal blocking (VBloc) therapy for the management of obesity (Anaes.) (Assist.) Fee: $334.25 Benefit: 75% = $250.70 MBS Item number XXXXX LEADS, flexible with electrodes, surgical placement of, for the management of obesity, to a maximum of 2 leads (Anaes.) (Assist.) Fee: $661.60 Benefit: 75% = $496.20 MBS Item number XXXXX NEUROREGULATOR, subcutaneous removal of, including removal and dis-connection of electrodes (Anaes.) (Assist.) Fee: $334.25 Benefit: 75% = $250.70 Notes: To refer to patient criteria and physician training requirements The proposal suggests that the proposed MBS items relating to the placement of a neuroregulator and placement of leads/electrodes most closely resemble placement of a neurostimulator (MBS item 39134) and placement of associated leads (MBS item 39138) for the management of chronic intractable neuropathic pain or pain from refractory angina pectoris. However, there are other items currently on the MBS which also relate to interventions involving the placement of a neuroregulator or similar device (neurostimulator or pulse generator) and associated services. These items are listed within Attachment A. Services relating to intra-abdominal vagal nerve modulation therapy proposed for funding on the MBS relate to i) placement of a neuroregulator, ii) placement of leads/electrodes and iii) removal of the neuroregulator and electrodes (note: leads are not specified). PASC noted that no MBS items relating to replacement of the neurostimulator and replacement of leads/electrodes were proposed despite the projection that the neurostimulator would need to be replaced approximately every 5 years and the leads/electrodes replaced every 15-20 years. PASC advised that it would be appropriate for a submission requesting subsidy of intraabdominal vagal nerve modulation therapy to include a request for MBS items for the replacement of the neurostimulator and replacement of the leads and electrodes. Wording of proposed listings PASC noted that the proposed MBS item descriptors include the term “VBLOC”, which is a trademark. PASC resolved that it would be more appropriate for the MBS items descriptors to include the generic term of “intra-abdominal vagal nerve modulation”. PASC noted that the proposed MBS items descriptors do not consistently include a description of both the intervention and indication. This could make it difficult for physician to readily identify service related to intra-abdominal vagal nerve modulation therapy. For example, the item relating to “LEADS” does not refer to leads that are used to deliver vagal nerve modulation therapy. In addition, the item relating to “NEUROREGULATOR, subcutaneous removal of…” does not refer to the indication (e.g., “for intra-abdominal vagal nerve modulation therapy used in the management of obesity”). PASC recommends that the item descriptors be amended to include a more precise description of the intervention and indication. Proposed fees The Schedule fees for the proposed items are derived on the basis of a claim that the proposed items are comparable to MBS item descriptors for placement of a neurostimulator and associated leads for the management of chronic intractable neuropathic pain or pain from refractory angina pectoris, namely MBS items 39134 and 39138, which have Schedule fees of $334.25 and $661.60 respectively. Table 4 provides a comparison of Schedule Fees for items that relate to placement and removal of the neurostimulator/pulse generator and also in fees for items that relate to placement and removal of associated leads and electrodes for other indications. Full details are provided in Attachment A. PASC noted that, for interventions involving the use of a neuroregulator, the fees for the removal of the neuroregulator are typically significantly lower (<50%) than the fee for placement of the neuroregulator. In contrast, the requested fee for removal of the neuroregulator for intra-abdominal vagal nerve modulation therapy is identical to the requested fee for the placement of the neuroregulator. PASC resolved that input from stakeholders should be sought on the proposed MBS fees and whether it was reasonable to assume that, technically, the placement of the neuroregulator and electrodes for intra-abdominal vagal nerve modulation was comparable to placement of a neuroregulator and electrodes used in the treatment of neuropathic pain. Table 4: Comparison of Schedule fees for MBS items relating to the placement, replacement, maintenance and removal of neurostimulators/pulse generators and associated leads and electrodes. Deep brain Obesity Neuropathic Faecal Urinary MBS service stimulation for (proposed) pain incontinence incontinence Parkinson’s Placement of neurostimulator/pulse $334.25 $334.25 $327.75 $327.75 $334.25 generator/neuromodulator Adjustment/programming of neurostimulator/pulse $125.40 $123.05 $123.05 $186.15 generator/neuromodulator Replacement or neurostimulator/pulse $250.70 $250.70 generator/neuromodulator Removal of neurostimulator/pulse $334.25 $156.45 $153.40 $153.40 $250.70 generator/neuromodulator Placement of leads/electrodes $661.60 $648.65 Replacement of leads/electrodes $594.05 $582.50 Removal of leads/electrodes $156.45 $153.40 Removal of neurostimulator and leads (combined) $661.60 $516.60 $598.90 $516.60 $516.60 $516.60 In relation to the fees for the surgical placement of the device, the proposal describes “as a rough guide a possible market price of between $3,000.00 and $4,000.00”. In addition to the fee for surgical placement of the device, the proposal suggests that the proposed market price for the device (The Maestro SystemTM) will be $17,850. PASC noted there will be a large difference between the Schedule fee for services relating to intra-abdominal vagal nerve modulation therapy and the fees charged in practice for the services. This will not have implications for Government budgets as a consequence of the Extended Medicare Safety Net as this Safety Net only applies for Medicare services received out-of-hospital whereas the services proposed are all delivered in hospital. However there are potential costs to Government as a consequence of the Government’s Net Medical Expenses Tax Offset (NMETO scheme). In 2012/2013 the scheme will permit taxpayers with an adjusted taxable income of less than $84,000 for singles (or $168,000 for a couple or family) to claim a reimbursement of 20% for net medical expenses over $2,120 (CPI indexed for 2012-13) when they lodge their tax return. It will also permit taxpayers with an adjusted taxable income of more than $84,000 for singles (or $168,000 for a couple or family, with the family threshold increasing by $1,500 for each dependent child after the first) to claim a reimbursement of 10% for eligible out of pocket expenses incurred in excess of $5,000. Furthermore, although there will be no implications for Government as a consequence of the Extended Medicare Safety Net in relation to the fees for surgical placement of the device, there may be implications for ancillary services that are provided in the community pre- and post-operatively. PASC considered that a large differential between the Schedule fee and the fee likely to be charged in practice raised potential issues of equity of access for patients. Medicare’s objectives are (i) to make health care affordable for all Australians; (ii) to give all Australians access to health care services with priority according to clinical need, and (iii) to provide a high quality of care 5. The MBS is one program administered by Medicare. The Department’s Annual Report for 2010-2011 states that the objective of the MBS is to provide access to high quality medical, dental and associated services to help people manage their health. This is achieved by (i) improving access to evidence based, best practice medical services; (ii) supporting access to necessary medical services that may not be available through mainstream mechanisms or which may not be available in Australia; (iii) supporting access to high quality, safe, clinically relevant and cost effective diagnostic imaging and pathology services; (iv) improving access to, and quality of, radiation treatment facilities for Australians with cancer; (v) providing targeted assistance to eligible people to access health care not covered under existing programs. A large price differential between the Schedule fee and the fee likely to be charged in practice means that intra-abdominal vagal nerve modulation therapy, if recommended for inclusion on the MBS with the proposed Schedule fees, would remain unaffordable, and therefore inaccessible, for numerous Australians (unless made available to patients managed through public hospitals). Thus, there is the potential for priority for treatment with intra-abdominal vagal nerve modulation to be determined on the basis of ability to pay. PASC noted that the reason for the difference between the proposed Schedule Fee and the market price is not explained in the proposal. As noted by the applicant, there is a differential between fees charged in practice and the MBS Schedule fee for other bariatric surgery interventions. As shown in Table 5, there are substantial differences between the fees charged in practice and the Schedule fee for other bariatric surgery items. Only around 6% of MBS services for gastroplasty (MBS Item 30511), gastric bypass and partial gastrectomy are bulk-billed. However, PASC noted that the differential proposed for vagal nerve modulation therapy was larger than that applying, on average, for other bariatric surgeries. PASC advised that any application requesting listing of services relating to intra-abdominal vagal nerve modulation therapy should provide a breakdown of fees likely to be charged in practice for the placement, replacement and removal of the neuroregulator and electrodes/leads and also for the device component. PASC resolved that stakeholder input should be sought on the implications of a large differential between the MBS Schedule fee and the fee likely to be charged in the market. Table 5: Comparison of average fees charged and MBS Schedule fee for MBS items relating to bariatric surgery for obesity MBS MBS Schedule Average fee Item MBS item descriptor fee charged1 number 30511 MORBID OBESITY, gastric reduction or gastroplasty for, by any method $833.70 $1,886 MORBID OBESITY, gastric bypass for, by any method including 30512 $1,025.90 $1,621 anastomosis 30518 PARTIAL GASTRECTOMY $969.10 $1,615 1 Source: Department of Health and Ageing 5 Source: http://www.medicareaustralia.gov.au/provider/medicare/index.jsp [Last accessed: 4 July 2012] Proposed population PASC noted that the proposed item descriptors (as shown in Table 3) do not include any restriction (beyond “obesity”) on the patient population that may access intra-abdominal vagal nerve modulation therapy. The 2003 Australian Clinical Practice Guidelines (currently under review) recommend bariatric surgery for adults with a BMI>40 or with a BMI>35 and serious medical comorbidity who have instituted but failed adequate non-operative measures for weight loss with integrated components of a dietary regimen, appropriate exercise, and behaviour modification and support 6. Therefore, the currently proposed indication of ‘obesity’ in the MBS item descriptor does not reflect the current clinical guidelines, introducing a risk that the service will be provided to subjects where the benefits have not been proven to outweigh the safety risk. PASC noted that although the requested listing does not include any restrictions (beyond “obesity”), the proposal suggests that intra-abdominal vagal nerve modulation therapy could be used in four potential target populations: 1. Patients with a BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with at least one co-morbidity; the proposal indicates that the majority of patients that would be treated with intra-abdominal vagal nerve modulation therapy would be in this classification; 2. Patients who are deemed unsuitable for the alternative Medicare Benefits Schedule (MBS) funded forms of bariatric surgery such as gastric banding or sleeve gastrectomy; 3. Women who intend, or who are likely, to become pregnant; 4. Patients who are super obese and need to lose some weight prior to gastric bypass. Issues that arise in relation to the requested listing and in identifying potential target populations include: An unrestricted listing may result in use of intra-abdominal vagal nerve modulation therapy in a broader population than included in the proposal’s description of the target population. For example, there is potential for the intervention to be used in patients who have failed to lose sufficient weight with other forms of bariatric surgery (i.e., there is potential for the intervention to be used in addition to currently available therapies). Furthermore, although there are currently guidelines that define the characteristics of patients who should be eligible for bariatric surgery, there is substantial debate around various aspects of those guidelines e.g., the BMI thresholds, the clinical circumstances in which bariatric surgery is indicated in adolescents. Even if surgeons conducting bariatric surgery complied with the eligibility criteria in the guidelines, if the listing for access to intra-abdominal vagal nerve modulation therapy were unrestricted, patients who would experience a potentially reduced benefit (compared with other patients who currently satisfy the criteria in the guidelines) would automatically become eligible for MBS-subsidised treatment if the guidelines were broadened to include more patients (e.g., patients with BMI ≥ 2 30 kg/m compared with the current requirements). The proposed listing does not include any restriction on the age of the patient (e.g., a limitation specifying that the patient is an adult), which would mean that the intervention could potentially be used in adolescents and children. PASC sought clinical expert advice about the suitability of the 6 Department of Health and Ageing (2003), Clinical practice guidelines for the management of overweight and obesity in adults, National Health and Medical Research Council (NHMRC) http://www.health.gov.au/internet/main/publishing.nsf/Content/obesityguidelinesguidelines-adults.htm, accessed 2 July 2010. procedure for patients under 18 years of age. The clinical expert advised that vagal nerve modulation therapy was currently not approved for use in adolescents or children and that best practice guidelines for obese under-18s indicate that gastric banding is the treatment of choice when bariatric surgery is indicated in patients aged >14 years. Unless advice to the contrary was received during the consultation phase of the development of this DAP, PASC considered that it would be appropriate for the MBS item descriptor to limit use of intra-abdominal vagal nerve modulation therapy to adults. There is a potential for variation in the extent of benefit likely to be gained by patients in the various populations (such that variations in incremental cost-effectiveness are possible); Some of the populations identified in the proposal are not mutually exclusive populations (e.g., patients who are super obese who need to lose some weight prior to gastric bypass are likely to be a sub-group of the population with a BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with at least one comorbidity. Some terms included in the proposal’s description of the target population may be inadequately specified. For example: o in relation to the subgroup of patients with a BMI ≥ 35 kg/m2 with at least one co-morbidity, it is notable that the clinical guidelines for bariatric surgery 7 suggest that the patient should have a “serious medical co-morbidity” (as opposed to any co-morbidity) in order to be considered a candidate for bariatric surgery; PASC agreed with the sponsor that the patient group most likely to use vagal nerve modulation therapy were patients with clinically severe obesity, defined as BMI ≥ 40 kg/m2, or between 35 kg/m2 and 40 kg/m2 where there are other major medical conditions such as high blood pressure or diabetes. PASC noted that MSAC, in November 2011, had supported a suggestion in a review of existing MBS items for bariatric surgery that the term “morbid obesity” in the current item descriptors for bariatric surgery items on MBS be replaced by the term “clinically severe obesity”. It was noted that MSAC considered that some degree of clinical judgement rather than specific BMI or other threshold indicators be considered to reduce the possibility of a perverse incentive for patients to inappropriately gain weight in order to meet threshold levels for access to subsidised treatment. o in relation to the population of patients who are deemed “unsuitable” for other forms of bariatric surgery that are reimbursed under the MBS, it is unclear how “unsuitable” should be defined. Furthermore, no explanation is provided as to why intra-abdominal vagal nerve modulation therapy would be appropriate in this sub-population; PASC considered that patients in this category would generally be unsuitable due to contraindication for laparoscopic surgery. Given that laparoscopic surgery was required to implant the device to deliver intra-abdominal vagal nerve modulation such that if bariatric surgery was contraindicated then intra-abdominal vagal nerve modulation would also be contraindicated, PASC could not foresee that such a patient group existed. PASC resolved that input should be sought during the consultation phase of the DAP development process to confirm PASC’s 7 Department of Health and Ageing (2003), Clinical practice guidelines for the management of overweight and obesity in adults, National Health and Medical Research Council (NHMRC) http://www.health.gov.au/internet/main/publishing.nsf/Content/obesityguidelinesguidelines-adults.htm, accessed 2 July 2010. supposition that there was unlikely to exist a patient group in whom other bariatric surgeries were unsuitable but where intra-abdominal vagal nerve modulation therapy was suitable. o in relation to the population who intend, or who are likely, to become pregnant, no requirements in terms of weight status are suggested nor are any parameters in relation to the likely timing of a potential pregnancy stipulated (e.g., actively attempting to become pregnant; intending to become pregnant in the next 3 years; intending to become pregnant at some point in their lifetime); the current description would suggest that all women ever likely to become pregnant should be eligible. It is notable that women of reproductive age constitute the vast majority of bariatric surgery patients in Australia; PASC noted the applicant’s proposal to make intra-abdominal vagal nerve modulation therapy available for women intending, or who are likely, to become pregnant. PASC interpreted this proposal as suggesting that intra-abdominal vagal nerve modulation therapy could be used to improve rates of fertility in obese women. PASC considered that the eligibility criteria recommended for bariatric surgery generally (i.e., patients with BMI ≥ 40 kg/m2, or between 35 kg/m2 and 40 kg/m2 where there are other major medical conditions such as high blood pressure or diabetes) were also appropriate for obese women experiencing difficulties with fertility. Therefore, PASC resolved that there was no need for a specific MBS item for women intending to become pregnant. o in relation to the subgroup of super obese patients who need to lose weight prior to gastric bypass surgery, there are some contradictions in the proposal (e.g., the applicant’s proposal [on p.19] states that super obese patients are not currently considered suitable for intraabdominal vagal nerve modulation therapy) and no definition of super obese (e.g., in terms of BMI or other parameters) is provided; furthermore, the clinical need for intra-abdominal vagal nerve modulation therapy before gastric bypass in this group of patients is unclear. PASC noted the applicant’s proposal to make intra-abdominal vagal nerve modulation therapy available for super obese patients (patients with BMI > 50 kg/m2) who need to lose some weight prior to gastric bypass. PASC noted that the proposal was inconsistent with the applicant’s statement (on p.19 of the proposal application [Part C]) that “… the patient population deemed suitable for gastric bypass is usually the super obese and these patients are not currently considered suitable for VBloc Therapy”. PASC resolved that it should seek input during the consultation phase of the DAP development process to clarify whether there was a clinical place for intra-abdominal vagal nerve modulation therapy in the super obese who need to lose some weight prior to gastric bypass. PASC also resolved to clarify that other bariatric surgery interventions would not be indicated in this population. In summary, PASC considered that a submission should focus on an MBS listing targeting adult patients with clinically severe obesity. Table 6 provides an example of possible modifications to the requested MBS item descriptors that clarify the intervention and the patient population. Table 6: Examples of alternate MBS item descriptions Category 3 - THERAPEUTIC PROCEDURES MBS Item number XXXX NEUROREGULATOR, subcutaneous placement of, including placement and connection of electrodes, for vagal nerve modulation therapy for the management of adult patients with clinically severe obesity (Anaes.) (Assist.) Fee: $334.25 Benefit: 75% = $250.70 MBS Item number XXXXX LEADS, flexible with electrodes, surgical placement of, for vagal nerve modulation therapy for the management of adult patients with clinically severe obesity, to a maximum of 2 leads (Anaes.) (Assist.) Fee: $661.60 Benefit: 75% = $496.20 MBS Item number XXXXX NEUROREGULATOR for vagal nerve modulation therapy, placed subcutaneously, removal of, including removal and disconnection of electrodes (Anaes.) (Assist.) Fee: $334.25 Benefit: 75% = $250.70 Notes: To refer to patient criteria and physician training requirements Clinical place for proposed intervention The proposal describes that the provision of MBS services relating to intra-abdominal vagal nerve modulation therapy should be limited to a hospital setting. PASC noted that some follow-up services would be delivered in the community setting using existing MBS items. The proposal does not comment on the potential for access issues for patients in rural and remote communities. For example, if the device can only be implanted in major hospitals and there is a necessity for adjustments to be conducted at the same centres then potential difficulties for access to services arise for patients located in rural and remote areas. The current clinical pathway for patients eligible for bariatric surgery as presented in the proposal is illustrated in Figure 1. The proposal states that patients with obesity (level not described) must first undertake more conservative or non-invasive methods of weight loss (e.g., behavioural or pharmacotherapy interventions). If unsuccessful, they become eligible for bariatric surgery. Figure 1, indicates that some subjects are ‘unsuitable’ (term not defined) for bariatric surgery (gastric banding and sleeve gastrectomy). In the current algorithm, there are no further treatment options available for these patients. The clinical pathway for “other forms of bariatric surgery especially gastric banding and sleeve gastrectomy” is described in the proposal as follows: Pre-surgical intervention for obesity, the patient would have tried many other alternatives to lose weight. Some, but not all, of these alternatives would have been suggested and/or under the supervision of a medical practitioner. These alternatives include: Un-assisted dieting (including fad diets) Weight Watchers or similar Pharmaceuticals – prescription and/or non-prescription Food replacement eg Optifast Personal trainer or Gym In the case of obese patients with co-morbidities such as diabetes, the patient will usually be under the care of an endocrinologist. The endocrinologist, or the patient’s GP (if not under the care of an endocrinologist), will refer the patient to a bariatric surgeon. Prior to the first consultation, most bariatric surgeons require that potential patients attend an information session on the various alternatives of bariatric surgery available, the associated advantages and disadvantages, risks and expected outcomes. The bariatric surgeon reviews the patient’s clinical history, especially in relation to conservative or non-invasive methods of weight loss (e.g., behavioural or pharmacotherapy interventions) and identifies any contra-indications for surgery. Standard blood tests are ordered for all patients and depending on the individual patient, other tests such as x-rays may be ordered. Figure 2 illustrates the clinical pathway in the hypothetical scenario that intra-abdominal vagal nerve modulation therapy is publicly funded. In this scenario, the population not suitable for currently available bariatric surgery procedures may be eligible for intra-abdominal vagal nerve modulation therapy. PASC noted that the management algorithms presented in the proposal suggest that intra-abdominal vagal nerve modulation therapy is to be positioned for use only in patients who are ineligible for currently available bariatric surgery procedures (and therefore provides a new therapy which satisfies a previously unmet clinical need). PASC noted that, given that the majority of use of intra-abdominal vagal nerve modulation therapy would be in patients with clinically severe obesity where it would compete with other bariatric surgery interventions (particularly gastric banding), then it would be more appropriate for the algorithm to position intra-abdominal vagal nerve modulation therapy alongside other bariatric surgery interventions in the management algorithm rather than following other bariatric surgery interventions as shown in Figure 3. Figure 1: Current clinical management algorithm (without intra-abdominal vagal nerve modulation therapy) Bariatric surgery is an option for obese patients when other conservative or noninvasive methods of weight loss (e.g., behavioural or pharmacotherapy interventions) have been unsuccessful. Sleeve Gastrectomy Gastric Banding Gastric Bypass Patient deemed to be ineligible for either of these procedures No alternate therapy Figure 2: Proposed clinical management algorithm (with intra-abdominal vagal nerve modulation therapy) Bariatric surgery is an option for obese patients when other conservative or noninvasive methods of weight loss (e.g., behavioural or pharmacotherapy interventions) have been unsuccessful. Sleeve Gastrectomy Gastric Banding Gastric Bypass Patient deemed to be ineligible for either of these procedures VBLOC Therapy Figure 3: Clinical management algorithm illustrating likely clinical positioning of intra-abdominal vagal nerve modulation therapy) Adult patients with clinically severe obesity who have failed treatment with conservative and non-invasive methods of weight loss (e.g., behavioural and pharacotherapy interventions) Sleeve gastrectomy Gastric banding Gastric bypass Intra-abdominal vagal nerve modulation therapy Comparator The proposal nominates comparators for three of the target populations. The populations and nominated comparators are summarised in Table 7. Table 7: Nominated comparators Target population Comparator BMI ≥ 40 or BMI ≥ 35 with at least one co-morbidity Other forms of bariatric surgery especially gastric banding and sleeve gastrectomy Patients who are deemed ‘unsuitable’ for the alternative MBS funder forms of bariatric surgery such as gastric banding or sleeve gastrectomy No surgery Women who intend, or who are likely, to become pregnant. (As per comments under “description” clarification is required regarding whether this group is severely obese) No surgery Patients who are super obese and need to lose some weight prior to gastric bypass No comparator nominated As discussed in the section titled “Proposed population”, PASC considered that the target population for intra-abdominal vagal nerve modulation therapy would be patients with clinically severe obesity, defined as BMI ≥ 40 kg/m2, or between 35 kg/m2 and 40 kg/m2 where there are other major medical conditions such as high blood pressure or diabetes. As discussed in the section titled “Clinical place for proposed intervention”, it is likely that the clinical position of intra-abdominal vagal nerve modulation therapy would be as a competitor to other types of bariatric surgeries. A range of bariatric surgery procedures are currently funded through the MBS and these procedures vary with regards to cost, efficacy, side-effects and acceptability to patients. Gastric banding is the most widely used bariatric surgery procedure in Australia8 and therefore may be the most appropriate comparator. Alternatively, a comparison versus a “basket” of bariatric surgery procedures (weighted by extent of use) could be conducted. Clinical claim The proposal claims that intra-abdominal vagal nerve modulation therapy is associated with an average excess weight loss (EWL) of at least 30%. Generally, ‘excess weight’ is usually calculated as weight (in kg) at the time of surgery minus ‘ideal’ or ‘desirable’ weight (in kg). Ideal/desirable weight is taken from a reference such as the revised Metropolitan Life height-weight tables9. Weight loss is then reported as a percentage of the excess weight (%EWL). It is not clear if the proposal is indicating that the results show an EWL greater than 30% or if the proposal is saying that the outcome that is to be reported is proportion of patients achieving an EWL >30% or if mean excess weight loss is to be reported and that a difference of >30% is clinically significant. 8 9 Source: Department of Health and Ageing, Draft report for reviewing existing MBS items. Available at http://www.health.gov.au/internet/main/publishing.nsf/Content/7C0B7F27D7F739B5CA25782A00821F3F/$File/DRAFT%20o besity%20report.pdf 1983 Metropolitan height and weight tables. Stat Bull Metrop Life Insur Co 1984; 64:2-9 In addition, it is claimed that there is a reduction in the prevalence of a number of co-morbidities, the main one being diabetes. Others include: cardiovascular disease; high blood pressure; stroke; high cholesterol; obstructive sleep apnoea; osteoarthritis, and some cancers. PASC noted that an association between weight loss and rates of remission of diabetes has been established for other bariatric surgery interventions. However, PASC noted that the relationship between weight loss and rates of these events could vary depending on the mechanism used to achieve weight loss e.g., remission from type 2 diabetes is known to vary with the type of bariatric surgery procedure (where gastric bypass has been shown to be associated with higher rates of resolution of type 2 diabetes than restriction only interventions such as gastric banding - several hypotheses have been postulated to explain these findings, including calorie restriction, hormonal changes, and exclusion of the upper gastrointestinal tract) 10. For this reason, PASC considered that it would be important for any application requesting the listing of services related to intra-abdominal vagal nerve modulation therapy to include evidence of impact on conditions that are thought to be related to obesity (e.g., diabetes, cardiovascular disease, etc). PASC noted that any assumption of a surrogate relationship between the endpoint of weight loss and reduction in risk of these other conditions should be supported by the presentation of evidence. PASC considered that it would not be appropriate to assume a relationship on the basis of data for other interventions as such an assumption would be fraught with a high degree of uncertainty. If such evidence to link the endpoint of weight loss with endpoints of other conditions is not available, it would be important for any application to outline how it intends to develop such evidence to permit review of any listing of intra-abdominal vagal nerve modulation therapy (e.g., establishment of a registry to capture long term outcomes or whether outcomes for this intervention will be captured by the registry of patients undergoing bariatric surgery being maintained by the Obesity Surgery Society of Australia & New Zealand [OSSANZ]). PASC noted that although it appears that the clinical claim to be made in the submission would be one of superiority of intra-abdominal vagal nerve modulation therapy in terms of comparative effectiveness relative to no treatment, it was not clear from the proposal what claim would be made in regards to comparative effectiveness and comparative safety of intra-abdominal vagal nerve modulation therapy relative to other bariatric surgery interventions. The clinical claim for intraabdominal vagal nerve modulation therapy versus other bariatric surgery interventions will determine the type of economic evaluation that should be presented in an application (see Table 8). 10 Tejirian et al. Bariatric Surgery and Type 2 Diabetes Mellitus: Surgically Induced Remission. J Diabetes Sci Technol. 2008;2(4):685-691 Table 8: presented Classification of an intervention for determination of economic evaluation to be Comparative safety versus comparator Superior Superior CEA/CUA Non-inferior CEA/CUA Comparative effectiveness versus comparator Non-inferior Inferior Net clinical benefit CEA/CUA CEA/CUA Neutral benefit CEA/CUA* Net harms None^ CEA/CUA* None^ Net clinical benefit CEA/CUA Neutral benefit CEA/CUA* None^ None^ Net harms None^ Abbreviations: CEA = cost-effectiveness analysis; CUA = cost-utility analysis * May be reduced to cost-minimisation analysis. Cost-minimisation analysis should only be presented when the proposed service has been indisputably demonstrated to be no worse than its main comparator(s) in terms of both effectiveness and safety, so the difference between the service and the appropriate comparator can be reduced to a comparison of costs. In most cases, there will be some uncertainty around such a conclusion (i.e., the conclusion is often not indisputable). Therefore, when an assessment concludes that an intervention was no worse than a comparator, an assessment of the uncertainty around this conclusion should be provided by presentation of cost-effectiveness and/or cost-utility analyses. ^ No economic evaluation needs to be presented; MSAC is unlikely to recommend government subsidy of this intervention. Inferior Outcomes and health care resources affected by introduction of proposed intervention Clinical outcomes The proposal describes that a reduction in obesity is expected due to intra-abdominal vagal nerve modulation therapy. A number of metrics could be used to report the extent of weight loss. The proposal appears to suggest that proportion of patients achieving various levels of EWL is a relevant metric. In addition, the proposal suggests that prevalence of the following health outcomes is also a relevant health outcome to consider in the determination of comparative effectiveness of interventions for obesity: diabetes; cardiovascular disease; high blood pressure; stroke; high cholesterol; obstructive sleep apnoea; osteoarthritis; and, some cancers. PASC advised that it was important that any application should also present a comparison of intraabdominal vagal nerve modulation therapy and other bariatric surgery interventions considering the following outcomes: rates of compliance with therapy (particularly in the context that the proposal states that the intra-abdominal vagal nerve modulation therapy device needs to be charged daily by the patient) maintenance of weight loss over long term horizons; impact on quality of life; impact on survival; incidence of safety-related events (e.g., rates of different types of complications; rates of infection; rates of reversal; rates of conversion from laparoscopic to open procedure; rates of revisional surgery and other major adverse events; rates of post-operative mortality); and incidence of adverse events secondary to vagal nerve inhibition Given the implied claim that intra-abdominal vagal nerve modulation therapy could be used to improve fertility rates in obese women of child-bearing age experiencing difficulties with becoming pregnant, PASC considered that outcomes in terms of rates of pregnancy should also be presented. PASC noted that the advice from the applicant that the intensity (amplitude) of the signal delivered to the vagal nerve is adjusted “according to patient tolerance” and that, typically, vagal nerve modulation is delivered for 12 hours per day (turned off at night because therapy is not required while patient is sleeping). PASC advised that any application requesting availability of services relating to intraabdominal vagal nerve modulation should provide any data available exploring whether there is a “dose-response” relationship between the strength and duration of the vagal nerve inhibition delivered and the outcomes achieved by a patient, including harms or adverse events associated with inhibition of the vagal nerve. Given the “newness” of this intervention, PASC agreed that it would be appropriate for narratives from a patient perspective, describing patient experience with the intervention, to be presented from a broad range of patients (including those who have stopped using the device) and presented with an application. Health care resources PASC reminded the applicant that the economic evaluation should take a health care perspective whereby costs borne by both the MBS, costs for the device, and out-of-pocket patient costs are included in the evaluation. The proposal claims that the healthcare resources used as part of intra-abdominal vagal nerve modulation therapy are very similar to those used for gastric banding or sleeve gastrectomy. It is also described that “the main differences, apart from the implanted prosthesis, are the duration of surgery, length of stay and post-operative care”. PASC suggested that the applicant should provide details in the application about the typical duration of surgery, the length of a typical hospital stay, and the typical follow-up post operatively and in the longer term both for intra-abdominal vagal nerve modulation therapy and for other bariatric surgery interventions. If differences are claimed, it is important that such claims are supported by presentation of evidence. Healthcare resources that may be used in the lead-up to a bariatric surgery procedure include: prior to surgery, some bariatric procedures require a low calorie diet (including food substitution products) for two to four weeks prior to surgery consultations with the bariatric surgeon (for the purposes of determining eligibility for bariatric surgery) consultations with a psychologist assessments (e.g., blood tests, radiological assessments) to determine a patients suitability for surgery Healthcare resources that may be used in the delivery of a bariatric surgery procedure include: operating theatre and associated resources – time taken varies depending on procedure services delivered by a bariatric surgeon services delivered by an assistant surgeon services delivered by an anaesthetist implanted device e.g., gastric band or staples hospital bed-days Healthcare resources that may be used following a bariatric surgery procedure include: consultations with the bariatric surgeon consultations with a medical practitioner to monitor weight loss and to monitor for potential adverse events consultations with a dietician consultations with a psychologist assessments (e.g., blood tests, imaging) to determine that a device has been correctly placed medications in the short term (e.g., analgesia) and long term (e.g., vitamin supplementation) band adjustments (gastric banding procedure only) adjustments to signal strength from the implanted device used to modulate the activity of the vagal nerve (intra-abdominal vagal nerve modulation therapy only) resources used to manage adverse events or complications some patients experiencing a substantial weight loss may be left with areas with excess skin which may require management by surgical resection; some patients experiencing a substantial weight loss may be able to undergo orthopaedic procedure that had previously been contraindicated due to the patient’s obesity some patients experiencing a substantial weight loss may have resolution of co-morbidities e.g., diabetes, hypertension, hypercholesterolaemia, sleep apnoea, which may result in a reduction in the use of therapies used to manage these conditions. Proposed structure of economic evaluation (decision-analytic) The following research question is suggested in the proposal: “What is the safety, effectiveness, and cost-effectiveness of intra-abdominal vagal nerve modulation therapy in the population of patients with BMI ≥ 40 or ≥ 35 with at least one comorbidity compared with other forms of bariatric surgery?” The proposal also describes “possible secondary questions” as follow: “What is the safety, effectiveness, and cost-effectiveness of intra-abdominal vagal nerve modulation therapy in patients for whom other forms of bariatric surgery are deemed to be unsuitable?” ‘What is the safety, effectiveness, and cost-effectiveness of intra-abdominal vagal nerve modulation therapy in female patients who have a high probability of becoming pregnant?” On the basis of its determinations in regard to the target population and clinical place for the intraabdominal vagal nerve modulation therapy relative to other interventions, PASC considered that the appropriate research question to be investigated in an application for intra-abdominal vagal nerve modulation therapy would be: “What is the comparative safety, effectiveness, and cost-effectiveness of intra-abdominal vagal nerve modulation therapy versus other bariatric surgery interventions in a population of adult patients with BMI ≥ 40 or ≥ 35 with at least one major co-morbidity?” The proposal does not outline a structure for a model (decision analytic) that could be used to conduct an economic evaluation comparing a scenario where intra-abdominal vagal nerve modulation therapy is available with the current scenario where it is not available. It will be important that the application present the structure of the decision analytic in both in a diagrammatic form and in a descriptive form (explaining in words what happens at the decision points and the transition points in the decision analysis). Decision and transition points in the diagrammatic representation of the decision analytic should be labelled and the written description of the decision analytic should include cross-references to the labelled points in the diagram summarising the structure of the economic evaluation. Ultimately, the presentation of the structure of the economic evaluation should make it apparent how the outcomes and health care resources identified as being important in determining the comparative clinical and economic performance of the proposed intervention versus the comparator(s) are incorporated into the economic analysis. This will permit the key drivers of the outputs (both costs and outcomes) of the economic analysis to be identified. The data sources for parameters in the model should also be specified. Fundamentally, the model will provide a comparison of intra-abdominal vagal nerve modulation therapy versus other types of bariatric surgery in adult patients with clinically severe obesity. The structure of the model that will be used to conduct an economic analysis should make apparent any relationships that will be accepted as applying between weight loss and other patient-relevant outcomes such as prevalence of co-morbidities. Although the proposal identifies some of the resources likely to be used prior to, in delivery of, and following placement of the device used for intra-abdominal vagal nerve modulation therapy, the proposal does not propose sources for the valuation each resource (i.e., the proposal does not identify sources for unit costs for resources e.g., the AR-DRG cost weight that applies for patients undergoing bariatric surgery is not identified. Although the proposal presented a list of resources to be considered in the economic analysis (as summarised in Table 9), PASC noted that the list of resources was incomplete. PASC noted the use of additional services following implant of the vagal nerve modulation device (e.g., consultations with physicians and allied health professionals such as dieticians, exercise physiologists and psychologists). It resolved that the use of these resources should be considered in the analyses of both the economic and financial implications of making the intervention available. Claims for differences in use of resources would need to be supported by presentation of evidence (e.g., claims that there will be a reduced need for routine monitoring by physicians would need to be supported with evidence demonstrating such a reduction). Table 9: List of resources to be considered in the economic analysis Provider of resource Setting in which resource is provided Number of units of resource per relevant Source of information time horizon per patient receiving of number of units* resource Resources provided to identify the eligible population that would vary from current clinical practice Resource 1 Resource 2, etc Surgeon Rooms once Pathologist Collection centre Standard bloods Resources provided in association with the proposed medical service to deliver the proposed Resource 1 Surgeon Theatre Approx one hour Resource 2 Assistant surgeon Theatre Approx one hour Resource 3 Anaesthetist Theatre Approx one hour Resources provided to deliver the comparator to deliver the current intervention Resource 1 Surgeon Theatre 45 to 90 minutes Resource 2 Assistant surgeon Theatre 45 to 90 minutes Resource 3 Anaesthetist Theatre 45 to 90 minutes Resources provided following the proposed intervention with the proposed medical service (from Step 8, e.g., resources used to monitor or in follow-up, resources used in management of adverse events, resources used for treatment of down-stream conditions conditioned on the results of the proposed intervention). Identify variations where these may vary across different decision options. Resource 1 Hospital Hospital One day Resource 2, etc Surgeon Rooms Twice Resources provided following the comparator to deliver the current intervention (from Step 7, e.g., resources used to monitor or in follow-up, resources used in management of adverse events, resources used for treatment of down-stream conditions conditioned on the results of the proposed intervention). Identify variations where there may be more than one comparator or where these may vary across different decision options. Resource 1 Hospital Hospital Two to four days Resource 2, etc Surgeon Rooms Once Attachment A MBS items relating to the placement and removal of neurostimulators or pulse generators and associated leads and electrodes Table 10 provides the MBS item descriptors for other services included on the MBS that involve the placement, management and removal of neurostimulators or other pulse generators and associated leads and electrodes. Table 10: Other MBS item descriptors relating to the placement, management and removal of neurostimulators or pulse generators and associated leads and electrodes Category 3 - THERAPEUTIC PROCEDURES ITEMS RELATING TO NEUROPATHIC PAIN MBS Item 39130 EPIDURAL LEAD, percutaneous placement of, including intraoperative test stimulation, for the management of chronic intractable neuropathic pain or pain from refractory angina pectoris, to a maximum of 4 leads (Anaes.) Fee: $661.60 Benefit: 75% = $496.20 MBS Item 39131 ELECTRODES, epidural or peripheral nerve, management of patient and adjustment or reprogramming of neurostimulator by a medical practitioner, for the management of chronic intractable neuropathic pain or pain from refractory angina pectoris - each day Fee: $125.40 Benefit: 75% = $94.05 85% = $106.60 MBS Item 39134 NEUROSTIMULATOR or RECEIVER, subcutaneous placement of, including placement and connection of extension wires to epidural or peripheral nerve electrodes, for the management of chronic intractable neuropathic pain or pain from refractory angina pectoris (Anaes.) (Assist.) Fee: $334.25 Benefit: 75% = $250.70 MBS Item 39135 NEUROSTIMULATOR or RECEIVER, that was inserted for the management of chronic intractable neuropathic pain or pain from refractory angina pectoris, removal of, performed in the operating theatre of a hospital (Anaes.) Fee: $156.45 Benefit: 75% = $117.35 85% = $133.00 MBS Item 39136 LEAD, epidural or peripheral nerve that was inserted for the management of chronic intractable neuropathic pain or pain from refractory angina pectoris, removal of, performed in the operating theatre of a hospital (Anaes.) Fee: $156.45 Benefit: 75% = $117.35 MBS Item 39137 LEAD, epidural or peripheral nerve that was inserted for the management of chronic intractable neuropathic pain or pain from refractory angina pectoris, surgical repositioning to correct displacement or unsatisfactory positioning, including intraoperative test stimulation, not being a service to which item 39130, 39138 or 39139 applies (Anaes.) Fee: $594.05 Benefit: 75% = $445.55 MBS Item 39138 PERIPHERAL NERVE LEAD, surgical placement of, including intraoperative test stimulation, for the management of chronic intractable neuropathic pain or pain from refractory angina pectoris, to a maximum of 4 leads (Anaes.) (Assist.) Fee: $661.60 Benefit: 75% = $496.20 Category 3 - THERAPEUTIC PROCEDURES ITEMS RELATING TO FAECAL INCONTINENCE MBS Item 32213 SACRAL NERVE LEAD(S), placement of, percutaneous using fluoroscopic guidance, or open, and intraoperative test stimulation, for the management of faecal incontinence in a patient who has an anatomically intact but functionally deficient anal sphincter with faecal incontinence refractory to at least 12 months of conservative non-surgical treatment (Anaes.) Fee: $648.65 Benefit: 75% = $486.50 MBS Item 32214 NEUROSTIMULATOR or RECEIVER, subcutaneous placement of, and placement and connection of extension wire(s) to sacral nerve electrode(s), for the management of faecal incontinence in a patient who has an anatomically intact but functionally deficient anal sphincter with faecal incontinence refractory to at least 12 months of conservative non-surgical treatment, using fluoroscopic guidance (Anaes.) (Assist.) Fee: $327.75 Benefit: 75% = $245.85 MBS Item 32215 SACRAL NERVE ELECTRODE(S), management, adjustment, and electronic programming of neurostimulator by a medical practitioner, for the management of faecal incontinence - each day Fee: $123.05 Benefit: 75% = $92.30 85% = $104.60 MBS Item 32216 SACRAL NERVE LEAD(S), inserted for the management of faecal incontinence in a patient who had an anatomically intact but functionally deficient anal sphincter with faecal incontinence refractory to at least 12 months of conservative nonsurgical treatment, surgical repositioning of, percutaneous using fluoroscopic guidance, or open, to correct displacement or unsatisfactory positioning, and intraoperative test stimulation, not being a service to which item 32213 applies (Anaes.) Fee: $582.50 Benefit: 75% = $436.90 MBS Item 32217 NEUROSTIMULATOR or RECEIVER, inserted for the management of faecal incontinence in a patient who had an anatomically intact but functionally deficient anal sphincter with faecal incontinence refractory to at least 12 months of conservative non-surgical treatment, removal of (Anaes.) Fee: $153.40 Benefit: 75% = $115.05 MBS Item 32218 SACRAL NERVE LEAD(S), inserted for the management of faecal incontinence in a patient who had an anatomically intact but functionally deficient anal sphincter with faecal incontinence refractory to at least 12 months of conservative nonsurgical treatment, removal of (Anaes.) Fee: $153.40 Benefit: 75% = $115.05 Category 3 - THERAPEUTIC PROCEDURES ITEMS RELATING TO URINARY INCONTINENCE MBS Item 36658 SACRAL NERVE STIMULATION for refractory urinary incontinence or urge retention, removal of pulse generator and leads Fee: $516.60 Benefit: 75% = $387.45 85% = $442.90 MBS Item 36660 SACRAL NERVE STIMULATION for refractory urinary incontinence or urge retention, removal and replacement of pulse generator Fee: $250.70 Benefit: 75% = $188.05 85% = $213.10 MBS Item 36662 SACRAL NERVE STIMULATION for refractory urinary incontinence or urge retention, removal and replacement of leads Fee: $598.90 Benefit: 75% = $449.20 85% = $525.20 MBS Item 36665 Sacral nerve electrode or electrodes, management and adjustment of the pulse generator by a medical practitioner, to manage detrusor overactivity or non obstructive urinary retention - each day Fee: $123.05 Benefit: 75% = $92.30 85% = $104.60 MBS Item 36666 Pulse generator, subcutaneous placement of, and placement and connection of extension wire(s) to sacral nerve electrode(s), for the management of a) detrusor overactivity; or b) non obstructive urinary retention that has been refractory to at least 12 months medical and conservative treatment in a patient 18 years of age or older. (Anaes.) Fee: $327.75 Benefit: 75% = $245.85 MBS Item 36668 Pulse generator, removal of, if the pulse generator was inserted to manage: a) detrusor overactivity; or b) non obstructive urinary retention that has been refractory to at least 12 months medical and conservative treatment in a patient 18 years of age or older. (Anaes.) Fee: $153.40 Benefit: 75% = $115.05 ITEMS RELATING TO DEEP BRAIN STIMULATION FOR PARKINSON’S DISEASE MBS Item 40852 DEEP BRAIN STIMULATION (unilateral) subcutaneous placement of neurostimulator receiver or pulse generator for the treatment of: Parkinson's disease where the patient's response to medical therapy is not sustained and is accompanied by unacceptable motor fluctuations; or Essential tremor or dystonia where the patient's symptoms cause severe disability. Category 3 - THERAPEUTIC PROCEDURES (Anaes.) (Assist.) Fee: $334.25 Benefit: 75% = $250.70 MBS Item 40856 DEEP BRAIN STIMULATION (unilateral) removal or replacement of neurostimulator receiver or pulse generator for the treatment of: Parkinson's disease where the patient's response to medical therapy is not sustained and is accompanied by unacceptable motor fluctuations; or Essential tremor or dystonia where the patient's symptoms cause severe disability. (Anaes.) Fee: $250.70 Benefit: 75% = $188.05 MBS Item 40862 DEEP BRAIN STIMULATION (unilateral) electronic analysis and programming of neurostimulator pulse generator for the treatment of: Parkinson's disease where the patient's response to medical therapy is not sustained and is accompanied by unacceptable motor fluctuations; or Essential tremor or dystonia where the patient's symptoms cause severe disability. (Anaes.) Fee: $186.15 Benefit: 75% = $139.65 85% = $158.25 MBS Item 40854 DEEP BRAIN STIMULATION (unilateral) revision or removal of brain electrode for the treatment of: Parkinson's disease where the patient's response to medical therapy is not sustained and is accompanied by unacceptable motor fluctuations; or Essential tremor or dystonia where the patient's symptoms cause severe disability. (Anaes.) Fee: $516.60 Benefit: 75% = $387.45 MBS Item 40858 DEEP BRAIN STIMULATION (unilateral) placement, removal or replacement of extension lead for the treatment of: Parkinson's disease where the patient's response to medical therapy is not sustained and is accompanied by unacceptable motor fluctuations; or Essential tremor or dystonia where the patient's symptoms cause severe disability. (Anaes.) Fee: $516.60 Benefit: 75% = $387.45