ONC_UCR__StructuredDataMtg_031111_FINAL
advertisement

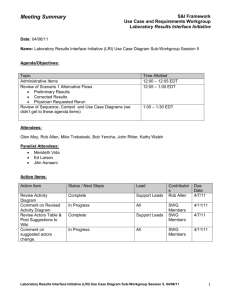
Meeting Summary ONC S&I Framework Use Case and Requirements Workgroup Date: 03/11/11 Name: Laboratory Results Interface (LRI) Structured Data Sub-Workgroup Session 2 Agenda/ Objectives: Structured Data Sub-Workgroup Purpose & Objectives Structured Data Discussion Next Steps Attendees: Panelist Attendees: Ed Larsen Merideth Vida Sub-Workgroup Attendees: Kosta Makrodimitris, Rob Hausam, Robert Dieterle, Ken McCaslin, Torin Shepard, Ken Willett, Hans Buitendijk, Nikolay Lipskiy, Andy Splitz, Carrie Adams, Action Items: Action Item Document Meeting Notes Next Steps / Status Complete Lead Merideth Vida Contributors Due Date N/A 3/15/11 Provide comments, revisions and suggestions on structured data definition using the wiki. Review background information from on defining and differentiating “structured data” from “standardized structured data” per the Final Rule. Coordinate with CDC & CMS on CLIA Issues Present Structured Data Definition to broader LRI UCR Workgroup In Progress All SWG Members 3/15/11 In Progress All SWG Members 3/15/2011 In Progress Jitin N/A Asnaani Kosta SWG Makrodimitris Members In Progress TBD 3/17/11 General Topics: The structured data definition was edited during the meeting using the wiki site. Structured Data Discussion: Defining decomposed LRI – Structured Data Sub-Workgroup Session 2, 03/11/11 1 Meeting Summary ONC S&I Framework Use Case and Requirements Workgroup o Structured data should go to lowest component, not compose in text strings, e.g., 25 mg should not be a single text entry but should be in two fields: test value, test unit of measure. o PCAST describes higher level of decomposition, e.g., a complete lab results report, whereas we are looking at the attributes within the result o A complete lab result must have context, not just result value, including identifiers, times, reference values Another issue of decomposition is ensuring that we specify not only the result as structured data but the lab test result name with data type and code/value set, e.g., lab test name from LOINC code set o Coordination between ONC and CDC needs Other: An additional structured data Sub-Workgroup may be scheduled for Wednesday March 16. The definition of structured data needs to be complete and presented to the LRI workgroup on 3/17/211 LRI – Structured Data Sub-Workgroup Session 2, 03/11/11 2