AP CHEMISTRY COURSE SYLLABUS 2012-2013
Periods 3,4
Brendan Haynie
brendan.haynie@boyd.kyschools.us
Text
Chemistry: The Central Science by Theodore L. Brown, H. Eugene LeMay, Jr., Bruce E. Bursten,
and Catherine J. Murphy, Pearson Prentice Hall, 12th edition, 2012.
Schedule
Listed below is the unit schedule for AP Chemistry. The first semester covers the first six units
while the second semester covers the remaining four units. Tests will typically cover multiple
units.
SUPPLIES: notebook, lab notebook (hard bound), graph paper, calculator
GRADING SCALE:
90-100
80-89
70-79
60-69
0-59
A
B
C
D
F
GRADING PROCEDURE:
1. TESTS - 50%
2. PROBLEM SETS - 15%
3. LAB REPORTS, consisting of purpose, procedure, data, data analysis, and conclusion 25%
4. TERM FINALS - 10%
TOPICS TO BE COVERED
Unit 1
Reading: Chapters 1-2 of Brown, LeMay, Bursten, and Murphy.
Topics: Welcome back to Chemistry (Atoms, Molecules, and Ions)
1. Atoms and the Periodic Table
2. Molecules and molecular compounds
3. Ions
Unit 2
Reading: Chapters 3-4 of Brown, LeMay, Bursten, and Murphy.
Topics: Stoichiometry and Predicting Reactions Products
1. Chemical equations
2. Patterns of chemical reactivity
3. Formula weights
4. Avogadro's number and the mole
5. Empirical formulas from analysis
6. Quantitative information from balanced equations
7. Limiting reactants
8. Properties of aqueous solutions
9. Precipitations reactions
10. Acid-base reactions
11. Oxidation-reduction reactions,
12. Concentrations of solutions,
13. Solution stoichiometry and chemical analysis
Labs:
1. Determination of the Empirical Formula of Silver Oxide
The determination of the percent composition and empirical formula of silver
oxide
2. Finding the Ratio of Moles of Reactants in a Chemical Reaction
The method of continuous variations is used to determine the mole ratio of two
reactants in an oxidation-reduction reaction
3. Liquid Chromatography
Liquid chromatography is used to separate the components of unsweetened
grape Kool-Aid
4. Gravimetric Analysis of a Metal Carbonate
The identity of a Group 1 metal carbonate is determined gravimetrically using a
double replacement precipitation reaction
Unit 3
Reading: Chapter 5 of Brown, LeMay, Bursten, and Murphy.
Topic: Themochemistry
1.
2.
3.
4.
5.
6.
What is energy?
First law of thermodynamics
Enthalpy and enthalpies of reactions
Calorimetry
Hess's Law
Enthalpies of formation
Labs:
1. Enthalpy of Reaction and Hess's Law
Hess’s Law is verified through application to three acid-base reactions and the
measurement of heats of reaction using a calorimeter
Unit 4
Reading: Chapters 6-7 of Brown, LeMay, Bursten, and Murphy.
Topics: The Electronic Structure of Atoms and Periodic Properties of the Elements
1. Wave nature of light
2. Quantized energy and photons
3. Bohr Model
4. Wave behavior of matter
5. Quantum mechanics and atomic orbitals
6. Many electron atoms
7. Electron configurations and the periodic table
8. History of the periodic table
9. Effective nuclear charge
10. Size of atoms and ions
11. Ionization Energy
12. Electron Affinities
13. Properties of metals, nonmetals, and metalloids
14. Trends for Groups 1A, 2A, 6A, 7A, and 8A
Labs:
1. Atomic Spectra and Atomic Structure
Examine the emission spectra for a series of Group 1A and Group 2A elements
2. An Activity Series
Determine the activity series for five metals and three halogens
Unit 5
Reading: Chapters 8-9 of Brown, LeMay, Bursten, and Murphy.
Topics: Chemical bonding and Predicting and Understanding Molecular Shapes
1. Chemical bonds
2. Lewis structures, and the octet rule
3. Ionic bonding
4. Covalent bonding
5. Bond polarity and electronegativity
6. Resonance structures
7. Exceptions to the octet rule
8. Strengths of covalent bonds
9. Molecular shapes
10. VSEPR model
11. Hybrid orbitals
12. Multiple bonds
13. Molecular orbitals and their application to diatomics and simple systems
Labs:
1. Molecular Geometries of Covalent Molecules
Examine the Lewis structures, VSEPR models, and three dimensional structures
of a series of simple covalently bonded molecules
2. Computational Models for Diatomics and Simple Systems
Use ab initio computational methods to compute molecular orbitals for a
selection of diatomics and simple polyatomic molecules
Unit 6
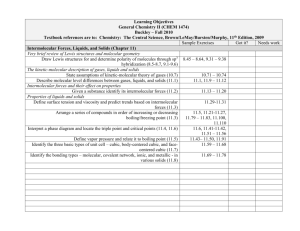
Reading: Chapters 10-11 of Brown, LeMay, Bursten, and Murphy.
Topics: Gasses, Liquids, and Solids
1. Pressure
2. Gas Laws (Boyle's Law; Charles's Law; Avogadro's Law)
3. Ideal Gas Equation
4. Molar Mass
5. Partial Pressure (Dalton's Law of Partial Pressures)
6. Kinetic-Molecular Theory
7. Effusion & Diffusion (Graham's Law)
8. Real Gases
9. Comparison of gases, liquids, and solids
10. Intermolecular forces & properties of liquids
11. Phase changes
12. Vapor Pressure
13. Phase diagrams
14. Structures and bonding of solids
Labs:
1. Determining the Molar Volume of a Gas
Determine the volume of one mole of H2 gas at STP
2. Determination of the Molar Mass of Volatile Liquids
Determine the molar masses of various volatile liquids
3. Analysis of Alum
Determine the melting point and mole ratio of hydrated water to anhydrous
aluminum potassium sulfate in AlK(SO4)2.12H2O
Unit 7
Reading: Chapters 13-14 of Brown, LeMay, Bursten, and Murphy.
Topics: Properties of Solutions and Chemical Kinetics
1. The solution process
2. Saturated solutions and solubility
3. Factors affecting solubility
4. Expressing concentration
5. Colligative properties
6. Colloids
7. Description of reactions rates and factors affecting reaction rates
8. The rate law and impact of concentration
9. Change of concentration with time (1st and 2nd order reactions)
10. Temperature and rate
11. Reaction mechanisms
12. Catalysis
Labs:
1. Molar Mass by Freezing Point Depression
Determine the molar mass of an unknown by measuring the freezing point
depression of a solution of the unknown and BHT
2. Preparation and Analysis of Tetraamminecopper(II) Sulfate Monohydrate
Synthesize Tetraamminecopper(II) Sulfate Monohydrate (one class period).
3. Kinetics of a Reaction
Determine total rate law for the oxidation of iodide ions by bromate ions in the
presences of acid
Unit 8
Reading: Chapters 15-16 of Brown, LeMay, Bursten, and Murphy.
Topics: Chemical Equilibrium and Acid-Base Equilibria
1. Concept of equilibrium and the equilibrium constant
2. Interpreting and working with equilibrium constants
3. Heterogeneous equilibria
4. Calculating equilibrium constants
5. Applications of equilibrium constants
6. Le Châtelier's Principle
7. Brønsted-Lowry acids and bases
8. Autoionization of water
9. pH scale
10. Strong acids and bases
11. Weak acids and bases
12. Relationship between Ka and Kb
13. Acid-base properties of salt solutions
14. Acid-base behavior and chemical structure
15. Lewis acids and bases
16. Expressing concentration
Labs:
1. The Determination of Keq for FeSCN2+
Determine the equilibrium constant for the reaction of Fe3+ and SCN2. The Determination of Ka for a Weak
To experimentally determine the pKa values for two weak acids
Unit 9
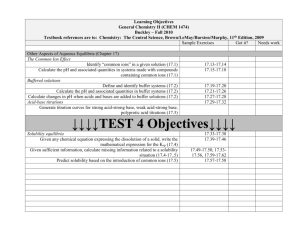
Reading: Chapters 17 and 19 of Brown, LeMay, Bursten, and Murphy.
Topics: Aqueous Equilibria and Chemical Thermodynamics
1. The common ion effect
2. Buffered solutions, acid-base titrations
3. Solubility equilibria, Ksp
4. Factors affecting solubility
5. Precipitations and separation of ions
6. Qualitative analysis for metallic elements
7. Spontaneous processes
8. Entropy and the second law of thermodynamics
9. Molecular interpretation of entropy
10. Entropy changes in chemical reactions
11. Gibbs free energy
12. Free energy and the equilibrium constant
Labs:
1. Acid Base Titrations
Standardize a solution of NaOH and then titrate an unknown acid
2. Preparation and Properties of Buffer Solutions
Prepare and investigate the properties of several buffer solutions
3. Separation and Qualitative Determination of Cations and Anions
Determine the cations and anions present in a solution by qualitative analysis
Unit 10
Reading: Chapters 20, 21, and 25 of Brown, LeMay, Bursten, and Murphy.
Topics: Electrochemistry, Nuclear Chemistry, and Organic Chemistry
1. Oxidation states and oxidation-reduction reactions
2. Balancing oxidation-reduction equations
3. Voltaic cells
4. Cell EMF under STP
5. Free Energy and Redox reactions
6. Cell EMF under nonstandard conditions
7. Batteries and fuel cells
8. Corrosion
9. Electrolysis
10. Radioactivity
11. Patterns of Nuclear stability
12. Nuclear transmutation
13. Rates of radioactive decay
14. General characteristics of organic molecules
15. Hydrocarbons
16. Alkanes, alkenes, and alkynes (structures and reactions)
17. Organic functional groups
18. Chirality in organic chemistry
Labs:
1. Analysis of Commercial Bleach
The amount of NaClO in commercial bleach will be determined
2. Electrolysis
Use an electrolysis cell to electrolyze an acidic solution of CuSO4
3. Synthesis of Aspirin and Wintergreen Oil
Synthesize acetylsalicylic acid and methyl salicylate using salicylic acid as our
starting point