pce12128-sup-0001-si
advertisement

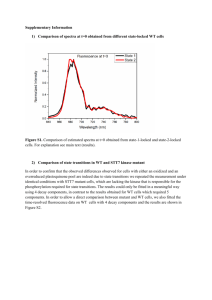
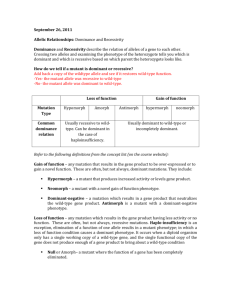
Supporting online material Materials and Methods Construction of the RNAi construct targeting the AtGOLLUM gene The binary vector pG3.3 was used for the RNAi experiment in A. thaliana. This homemade vector is derived from pBin19 (Bevan, 1984) and carries a CaMV35S-gus construct plus a second CaMV35S promoter for expression of a gene of interest. The antisens strand was obtained from a pGEM-Teasy vector carrying the AtGOLLUM cDNA obtained by RT-PCT with the oligonucleotides Narf500 and Narf300. The first 622pb of the cDNA were removed by NdeI restriction from the NdeI site of the pGEM-Teasy vector to the NdeI site of the AtGOLLUM cDNA. This deletion allowed the subsequent cloning of the ΔNdeI cDNA fragment (bp 623 to 1506) in the pG3.3 vector in the antisens orientation as an EcoRI/SacI fragment. The sens strand was obtained from a pGEM-Teasy vector carrying the genomic DNA of the AtGOLLUM gene amplified with the oligonucleotides Narf500 and Narf300. It was cloned as an XbaI-BamHI fragment (bp 14 to 2140 of AJ417911) in the pG3.3 vector carrying the antisens strand. The stability of the RNAi construct in the bacteria used for cloning and transformation was obtained by cloning a BamHI fragment containing the chloramphenicol gene of plasmid pSKS114 (Shapira et al., 1983). The spacer used in this construct (BamHI chloramphenicol fragment) was not spliced and resulted in the production of a double strand RNA with a RNA loop. Construction of the AtGOLLUM-GFP fusion Translational C-terminal fusions with GFP to AtGOLLUM were done by cloning the cDNA of the AtGOLLUM in the pSMrsgfp vector (Accession n° U70496). The cDNA was modified by PCR with the oligonucleotides NarfM5 introducing an XbaI restriction site (5’CCTCTAGAAAGCAACATGTCAGAGAAGTTTTCAC-3’) and NarfM3 introducing a BamHI restriction site (5’-GGGGATCCCAGGACCCCAGTTGTTGAGCTGCGA-3’) underlined in the oligonucleotides sequences. The NarfM3 oligonucleotide contains the last 6 codons of AtGOLLUM protein and converts the stop codon into a glycine in order to construct the GFP fusion. The PCR-amplified product was sequenced and cloned as an XbaIBamHI fragment in the pSMrsGFP carrying the 35S promoter. The binary vector used for the stable transformation of the fusion was constructed by introducing the AtGOLLUM-GFP fusion as an EcoRI-XbaI fragment in the pCAMBIA1390::p35S used for the complementation. GFP imaging Two-week-old plants were observed under confocal microscope (Leica SP2 Confocal microscope). The GFP fluorescence was monitored with a band pass filter (488nm excitation line of a Krypton/Argon laser). Typically 10-20 focal sections were obtained and the final image was obtained by the addition of the section with the LEICA Confocal Software. Silique Observation For silique observation and seed counting, green 2cm long siliques were harvested and cleared in Ethanol solution (96%) and observed under light macroscope (LEICA). Structural modeling and Amino-Acid variability The sequence alignment of 81 proteins related to IOP/GOLLUM/NAR family (including plant, fungi, fish, mammal and insect) was performed using the clustalX software. The different amino acids are classified by chemical properties (hydrophobic aliphatic AGILV, hydrophobic aromatic FW, amide NQ, hydroxil aromatic Y, hydroxyl polar ST, sulfur CM, dibasic RKH, diacid DE). Frequency of each class has been measured for each position. Amino acids position was excluded if the position was found less than 50% in the 81 protein used for alignment. The frequencies for conserved position were summed with a ponderation factor (32x if the frequency was >90%, 16 if >80%, 8 if >60%, 4 if >40%, 2 if >20% and 1 if >0%). A mean value (centered-window of 5 a.a.) has been used for the graphical representation. The modeling structure of AtGOLLUM, HsIOP1, HsIOP2 and ScNAR1 has been performed with the SPDBviewer software (http://spdbv.vital-it.ch/) using the X-Ray structure of the Clostridium pasteurianum Fe-only Hydrogenase (PDB code 1FeH). TABLE Table S1 : Transcriptomic analysis of the Atgollum mutant List of genes differentially expressed genes in the mutant sample were analyzed using WT sample grown in the same conditions as references. A negative ratio indicates that gene is down-regulated in mutant; a positive ratio indicates that the gene is up-regulated in mutant. Absent in these Excel files: gene not found to be statistically significant after Bonferroni correction (P<0.05) or FDR correction (P<0.05). Table S2: Metabolomic analysis of the Atgollum mutant The values are the ratio and log2 transformed ratio of the 82 measured metabolites in atgollum mutant compare to the metabolites in WT line. FIGURES: Figure S1: Construct of the binary vector for RNAi experiment against AtGOLLUM Restriction enzymes are indicated by their initials (E : EcoRI, X : XhoI, B : BamHI, Nd : NdeI, S : SstI). The use of the resistance cassette to chloramphenicol in this construct aimed at avoiding homologous recombination between forward and reverse strand of the AtGOLLUM gene. Figure S2: Expression of MtGOLLUM1, MtGOLLUM2 and AtGOLLUM in micro-array compendium.(a) Gene atlas expression of Arabidospis AtGOLLUM in various plant tissues. Gene expression of Medicago MtGOLLUM1 (b) and MtGOLLUM2 (c) in various plant tissues. The data were retrieved from Arabidopsis eFP browser (http://bar.utoronto.ca/efp/cgibin/efpWeb.cgi) and Medicago eFP browser (http://bar.utoronto.ca/efpmedicago/cgi- bin/efpWeb.cgi). The colour code is given for each panel Figure S3: Localization of the AtGOLLUM-GFP fusion in A. thaliana transgenic plants. The root apex of two-week-old in vitro plants was observed using the confocal microscope in order to avoid the chlorophyll auto-fluorescence. (a) Transgenic root expressing the AtGOLLUM-GFP fusion. A low level of fluorescence is detected in the cytoplasm and nucleus. (b) Transgenic root expressing the cytoplasmic GFP. With this construct the GFP is localized in the cytoplasm and in the nucleus. Scale bar: 40μm. (c) Onion epidermal cell transiently expressing the AtGOLLUM-GFP fusion (24hours after bombardment). Fluorescence can be detected throughout the cytoplasm and the nucleus. Scale bar: 40μm (d) Northern blot analysis of the stable transgenic lines transformed with GFP fusion and with the cytoplasmic GFP. For each sample, 10μg of total RNA was loaded on the gel. From left to right, lanes 1 to 8: transgenic lines expressing the AtGOLLUM:GFP and lanes 9 to 11 transgenic lines expressing the cytoplasmic GFP. Only plants from lane 10 and 11 expressed this cytoplasmic GFP. Note that the expression level is higher in these two lines as compare to the AtGOLLUM-GFP lines. Bottom panel: Methylene blue staining of the corresponding Northern blot. Figure S4: Structural modeling and protein variability. (a) Graphical representation of amino acid variability in IOP/NAR/GOLLUM family. The height of the peaks represents the levels of conservation of the protein. The regions with a 0 value correspond to insertion restricted to few species. The red colored peaks correspond to the hyper-variable region and the blue-colored peaks to the regions with the conserved cysteine residues involved in the FeS cluster binding. (b) Structural model of the ScNAR1, HsIOP1, HsIOP2/NARF and AtGOLLUM. The protein is represented as a ribbon diagram excepted for the hypervariable loop (backbone with sidechains). Color code for the side chain amino acids: red stand for negatively charged a.a., grey hydrophobic ones, blue positively charged ones, yellow neutral polar ones. The FeS clusters of CpFeH represented as red dots have been placed at the putative binding site of the IOP/NAR/GOLLUM protein. Figure S5: The homozygous knock out Arabidopsis gollum mutant is embryo lethalBox and Whisker plot of (a) number of available positions per silique in WT (white box) and heterozygous gollumT-DNA mutant (grey box) (b) number of unoccupied positions (c) the number of seeds per siliques (n=25 siliques for 5 independent offspring). Double asterisks indicate a statistically significant difference between WT and mutants (Mann-WhitneyWilcoxon’s Test, p<0.001). (d) Cleared siliques of heterozygous line (top) and WT line (bottom) showing the presence of empty spaces in the silique of heterozygous plants. The light photography shows a close-up view of the inner part of the silique of heterozygous line. Aborted seeds could be observed (white arrow). Figure S6: Silencing of the AtGOLLUM gene. (a) One-month-old T1 primary transformants. 12 independent lines transformed with the empty vectors are shown on the upper part. The 12 RNAi lines (bottom part) were numbered from At1 to At12. (b) Progeny of At1 (T2 generation) and At7 (T3 generation) RNAi lines. The progeny with dwarf phenotype possess the T-DNA carrying the RNAi construct (At7-1 to 3 and At1) whereas the line with WT phenotype (At7-4) did not carry the T-DNA. 26.6% of the progeny (169 plants out of 636 seedlings) showed a WT phenotype and 73.4% (467 plants out of 636 seedlings) the dwarf and stressed phenotype that indicates a dominant phenotype (2(dof=1)= 0.84; p<0.05). Scale bar: 1 cm. Figure S7: Description of the gollum1 mutant: flower morphology and in vitro growth. (a) Morphology of WT flowers from green house grown plants. (b) Morphology of gollum1 mutant flower from green house grown plants. (c) In vitro one-month-old plants (left:WT; right gollum1). (d) Greenhouse one-month-old plant (left:WT; right gollum1). (e) In vitro morphology of WT leaves. (f) In vitro morphology of gollum1 leaves. Note that the leaf morphology of the in vitro grown mutant plants is not affected. Scale bar: 1cm. Figure S8: expression of the 35S::AtGOLLUM chimeric gene in the transgenic lines. Top panel: amplification of the AtGOLLUM gene using the oligonucleotide couple Narf5/TerNOS. Bottom panel: amplification of the CAB gene using the oligonucleotide couple CAB5/CAB3. Lane 1: Molecular Weight marker (MW). Lane 2 to 8: transgenic lines transformed with the vector expressing the AtGOLLUM gene. Lane 9: wild-type plant. Lane 10: negative control. Note that lane 4 corresponds to a transgenic line that did not express the AtGOLLUM gene. This line retained the mutant phenotype indicated by the minus symbol. The PCR product of higher size observed lane 4 corresponds to the amplification of genomic DNA. CAB: Chlorophyll A Binding protein. Figure S9 : GO annotation of significant up-regulated genes in At7 compared to WT line. The search for enriched functional categories was done with the significant up-regulated genes (FDR correction method). The significant enriched functional categories are indicated by a color scale from white (no enrichment) to red (enrichment). The p-value in each box indicates the confidence for enrichment. High level of sucrose has an impact on anthocyanin biosynthesis and hydric stress response. Figure S10: GO annotation of significant down-regulated genes in At7 compared to WT line. The search for enriched functional categories was done with the significant down-regulated genes (FDR correction method). The significant enriched functional categories are indicated by a color scale from white (no enrichment) to red (enrichment). The p-value in each box indicates the confidence for enrichment. The functional category of biotic stress response shows enrichment in the down-regulated genes in mutant compare to WT. Figure S11: Statistical analysis of the metabolome profile of WT and gollum mutant. (a) Principal Component Analysis (PCA) on the log2 transformed value of the metabolite measurement. The WT replicates groups together separately of the mutant replicates. 80% of the observed variability is explained by the differences between WT and mutant. (b) PCA with the same dataset but focused on the metabolites. The difference observed between the two conditions is mainly explained by the difference observed in Proline and Sucrose content (indicated by red boxes) (c) Hierarchical clustering with metabolites showing significant differences between WT and mutant (p-value < 0.05). The T-test used for statistical analysis did not retain proline in this analysis. (d) Mean comparison for sucrose and proline content in WT (white bars) and mutant (grey bars) line. Figure S12 : Starch accumulation in WT and At7 at 21kPa O2. Lugol staining of leaves of one-month old Arabidopsis WT (a) and At7 mutant (b). The red colour of At7 leaves correspond to anthocyanin accumulation. Lugol staining of leaves of two-month old Medicago WT (c) and gollum1 mutant (d). Starch is coloured by lugol staining. Leaves are cleared by immersion into ethanol 96% solution. Scale bar : 1cm. Figure S13: Ploidy level of Arabidopsis and Medicago leaves and Medicago nodules at 21kPa O2 (a) Ploidy distribution in WT leaf (white bars), At5 (grey bars) and At7 (dark grey bars) in A. thaliana. Values are means ± SD of 5 independent leaves of two-weeks-old plants. Asterisks indicate a statistically significant difference from WT (Kruskal Wallis one way ANOVA, p<0.01 with Dunnett Post-Hoc analysis). (b) Ploidy distribution in WT leaf (white bars), gollum mutant (grey bars) and complemented gollum mutant (dark grey bars) in M. truncatula. Values are means ± SD of 10 independent leaves of two-monthd-old plants of R108-1 WT and gollum mutant. Asterisks indicate a statistically significant difference from WT (heteroscedastic Student’s t test, p<0.05). (c) Ploidy distribution in WT nodule (white bars) and gollum mutant (grey bars) in M. truncatula. (means, n=2). Figure S14: Recovery after mild hypoxic treatment in Medicago. Plants were initially grown at 5kPa O2 during one month and transferred at 21kPa O2 for two weeks. Leaves of the same plant were harvested and phenotypically screened. gollum1 leaves grown at 5 kPa O2 are similar to the WT, whereas the leaflets of the newly formed leaf (grown at 21kPa O2) show the mutant phenotype. Figure S15: Root growth in response to paraquat in normoxia (21kPa O2). Growth of Medicago WT (white bar) and gollum (grey bar) root on paraquat containing medium. 3 days old plants were transferred to GM medium containing increase concentration of paraquat (or Methylviologen) and the length of the root was measured 0, 3, 5 and 6 days after transfer. Root growth in presence of paraquat was compared against those grown in its absence. Bevan M. (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res, 12, 8711-8721. Shapira S.K., Chou J., Richaud F.V. & Casadaban M.J. (1983) New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene, 25, 71-82.