Do you see what I see? June 2012 Lesson 3: Is seeing believing
advertisement
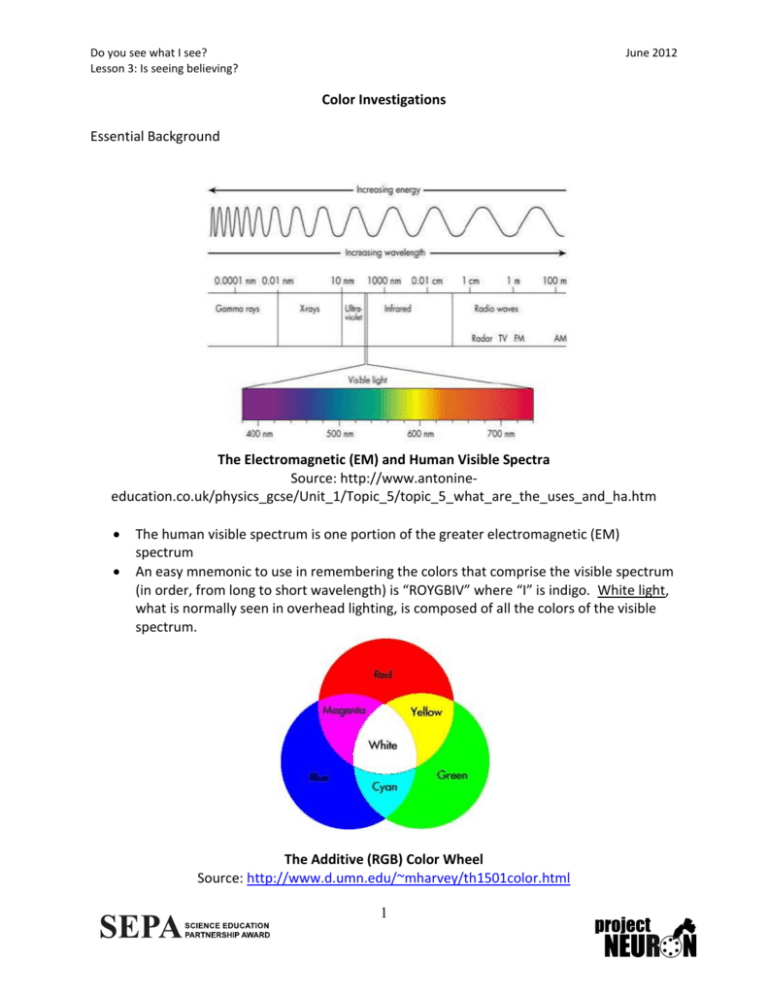
Do you see what I see? Lesson 3: Is seeing believing? June 2012 Color Investigations Essential Background The Electromagnetic (EM) and Human Visible Spectra Source: http://www.antonineeducation.co.uk/physics_gcse/Unit_1/Topic_5/topic_5_what_are_the_uses_and_ha.htm The human visible spectrum is one portion of the greater electromagnetic (EM) spectrum An easy mnemonic to use in remembering the colors that comprise the visible spectrum (in order, from long to short wavelength) is “ROYGBIV” where “I” is indigo. White light, what is normally seen in overhead lighting, is composed of all the colors of the visible spectrum. The Additive (RGB) Color Wheel Source: http://www.d.umn.edu/~mharvey/th1501color.html 1 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Humans possess “green,” “red” and “blue” cone cells Green, blue and red can be considered “primary physiological colors” Yellow, cyan and magenta can be thought of as “secondary physiological colors”; each results from the simultaneous and equal activation of two kinds of cone cells The perception of white results from simultaneous and equal activation of all three kinds of cone cells “Complementary physiological color pairs” are blue & yellow, red & cyan, and green & magenta. An object of color X maximally absorbs light at the wavelength of its complementary color Non-primary and non-secondary colors (e.g., orange, grey and brown) result from simultaneous and unequal activation of multiple cone cell varieties 2 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Monochromatic/White Light Emission Instructions: Use as many of the below graphs as needed to sketch the spectra generated by each light bulb in the experiments you do as a class. Label each graph. Light Bulb Used: Notes: 3 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Light Bulb Used: Notes: . 4 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Light Bulb Used: Notes: 5 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Monochromatic/White Light Emission: Questions 1. What happens to the graph when the probe is moved closer/further to/from the light source? 2. Why were the room lights dimmed before the spectrophotometer was used? 3. If the white light bulb used isn’t truly full-spectrum, why do you suppose we perceive the light it emits as white? 6 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Emissions from Multiple Lights Instructions: Use as many of the below graphs as needed to sketch the spectra generated by combinations of light bulbs in the experiments you do as a class. Label each graph. Your sketches need not be precise. Based on your class discussion, answer the questions below. Light Bulbs Used: Notes: 7 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Light Bulbs Used: Notes: 8 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Light Bulbs Used: Notes: 9 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Emissions from Multiple Lights: Questions 1. Do you and the spectrophotometer always “see” the same thing? What does this suggest about visual perception vs. optics? Use specific examples form the spectra generated in this part of the lesson. 10 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Reflection Instructions: Use as many of the below graphs as needed to sketch the spectra generated by light reflected off of objects in the experiments you do as a class. Label each graph. Based on your class discussion, answer the questions below. Light Bulb Used: Object Used: Notes: 11 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Light Bulb Used: Object Used: Notes: 12 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Light Bulb Used: Object Used: Notes: 13 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Reflection: Questions 1. Do the reflected spectra change based on how you position the probe relative to both the light source and the object? 2. How would you confirm that the spectrophotometer is measuring the light reflected from the object, and not merely recording the emission spectrum from the light source? 3. Is the reflected spectrum generated by shining a given color of light on, for example, a yellow M&M the same as the spectrum generated from reflecting that same color of light off of a yellow Christmas tree ornament? What difference does the nature of the material make? 14 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Absorption/Transmission Instructions: Use as many of the below graphs as needed to sketch both the absorbance and transmittance spectra generated by each liquid. Label each graph. Based on your class discussion, answer the questions below. Type of Spectrum: Color of Liquid: Notes: 15 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Type of Spectrum: Color of Liquid: Notes: 16 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Type of Spectrum: Color of Liquid: Notes: 17 Do you see what I see? Lesson 3: Is seeing believing? June 2012 Absorption/Transmission: Questions 1. Does a secondary color like yellow only transmit yellow light? Why might it transmit light in other regions of the spectrum and still appear yellow? 2. How do the absorbance and transmittance spectra for each color compare to each other? a. Red b. Blue c. Green d. Yellow 3. How do you imagine the darkness of the color would affect transmittance and absorbance? 18









