Brassica Covercrops for Honey Bees
advertisement

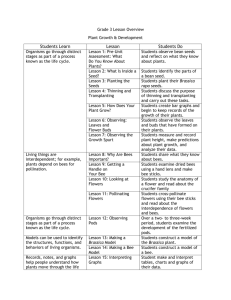
Brassica Cover Crops for Honey Bees Project Apis m Stephen Peterson Brassica is a genus in the plant family Brassicaceae (mustards). Some important food crops in the genus include broccoli, cabbage, Brussels sprouts, cauliflower, turnip, collard greens, rapini and mustard seed. This genus originates in Western Europe, the Mediterranean region and temperate regions of Asia (“Brassica,” 2013). Most Brassica grow well in cool climates or in the cooler part of the year in warm climates. Little auto-pollination occurs in most species and cultivars, and insect pollination is essential to produce good crops of seed (Free, 1993). The flowers of most Brassica plants are attractive to honey bees. Several Brassica species can be used for cover crops because they grow rapidly, provide erosion control, produce large amounts of biomass (up to 8,000 lb./A) and are excellent at scavenging nutrients (up to 140 lb. of nitrogen/A) (Cavanaugh, 2013). Brassica plants produce glucosinolates (GSLs) which the plants produce as a chemical defense against herbivores, plant pathogens and weeds. GSLs are hydrolyzed in the soil to form isothiocyanates (ITCs). These ITCs can inhibit weed seed germination and microorganisms in the soil. Canola Canola is rapeseed that has been bred for low levels of GSLs and erucic acid. The name “canola” was derived from the words “Canadian oil, low acid.” Canola may be B. napus or campestris and both are herbaceous annuals. Oil from the seeds of canola is used for cooking oils and biodiesel. If weed and pathogen suppression is desired from the cover crop, then rapeseed should be used instead of canola (Cavanaugh, 2013). Rapeseed can tolerate a wide range of soil conditions (pH from3.8 to 7.8) and can tolerate moderate salinity (Chapman and Carter, 1976). Canola should be planted at 5-10 lb./A no deeper than ¾ inch or broadcast at 8-14 lb/A (Cavanaugh, 2013). 1 Mustards Mustards are a group of species that include white or yellow mustard, B. hirta or Sinapis alba, brown or Indian mustard B. juncea, and black mustard B. nigra. All of these have high levels of GSLs. White mustard is commonly grown in vineyards in Napa and Sonoma counties and blooms from February to March there. A comparison of various Brassicas showed that B. juncea var. Pacific Gold coupled high biomass yield with above-average GSL production (Antonious et al., 2009). Black mustard can grow up to 8 feet tall, has indeterminate growth and with adequate moisture can bloom for several weeks. Mustards should be planted at 5-12 lb./A, ¼ to ¾ inch deep or broadcast at 12-20 lb./A (Cavanaugh, 2013). Rapini Rapini (B. rapa, the same species as turnip) is grown for its for its edible leaves (like turnip greens) and buds. It grows to about 2 feet tall with abundant yellow flowers and is very attractive to honey bees. It should be planted in the fall in California at 4-7 lb./A about ½ inch deep or broadcast at 10-12 lb./A (Cavanaugh, 2013). When planted in late October in the central valley of California it will bloom for 5 to 6 weeks, from late February to late March without irrigation. Benefits to the Soil and Subsequent Crops Fertility: Brassica cover crops grow rapidly in the fall and are excellent at scavenging nitrogen and other nutrients remaining in the soil after harvest. The crop can then be mowed and the residue can provide nutrient-rich mulch or disked in as green manure. Some microorganisms nitrify fertilizers, and the presence of Brassica crop residue can suppress these microorganisms, making subsequent nitrogen applications more available to plants (Brown and Morra, 2009). Even when no additional nitrogen is added to soils with Brassica crop residues, more nitrogen was available for plants compared with a grass cover crop. 2 Soil compaction: Some Brassica species (forage radish, rapeseed, turnip) have a taproot that can penetrate up to 6 feet and can help alleviate soil compaction. As the roots decompose, they leave channels that can improve water infiltration (Cavanaugh, 2013). Weeds: ITCs can reduce weed seed germination. In a study with winter rape, B. napus, weed biomass was reduced 96% when the cover crop was cut and allowed to decompose in the soil (Masiunas and Eastman, 2012). Plant Pathogens: ITCs are also toxic to some plant pathogens. Larkin and Griffin (2003) showed that Brassica cover crops grown as green manure reduced inoculums levels of Rhizoctonia, and the incidence of powdery scab and black scurf in potatoes. Some Brassica species can reduce populations of parasitic nematodes such as the root-knot nematode, as well (Monfort et al., 2007). Benefits for Honey Bees Brassica pollen is sticky, unlikely to be windborne and the flowers are highly attractive to honey bees. Honey bees readily collect nectar and pollen from two species of canola (Mohr and Jay, 1988) and spend 4.6 to 7.0 seconds per flower. The sugars in canola nectar do not contain sucrose, but have large amounts of glucose compared to fructose, indicating that the resulting honey will tend to granulate (Kevan et al., 1991). Canola produces abundant nectar, and can be more attractive than red or white clover (Hammer, 1966). Mustard flowers are also highly attractive to honey bees (McGregor, 1976). Because many Brassica species grow and bloom in the cool climates or seasons, the plants may come into bloom at temperatures below 55°F, making them unavailable to honey bees at those temperatures (McGregor, 1976). Canola, B. campestris, produces an average of 0.16 µl (0.28 mg) of nectar with 40% sugar concentration per flower per day which equates to 8.6 lb. of sugar per acre, whereas B. napa, Swede rape, produces 0.45 mg per flower per day or 87-207 lb. per acre. (Szabo, 1982, 1985). Cabbage nectar produces 12.6-15.5 lb. of sugar per acre and kohlrabi produces 33-47 lb. per acre (Free, 1993). A study 3 on Canola showed that flowers were visited on average 26 times per day, with visits beginning at 10 am. Visits reached a peak between noon and 2 pm, with no pollen in the flowers by 3 pm (Mohammed, 1935). Honey bees foraging on B. nigra and B. rapa, spent an average of 175-200 seconds per trip, with 4.2-4.6 seconds per flower (Collins et al., 1997). The flowers contained 0.15-0.23 mg/pollen and the pollen loads collected by the bees varied from 4-5 mg. Extrapolating from this data, assuming a forager takes 7 minutes per round trip (200 seconds foraging and 240 seconds in the hive), and foraging lasts from 10 am to 2 pm, or 4 hours, a forager could make 34 trips in a day. If a hive had 5,000 foragers and each trip brought in 4 mg of pollen, this would amount to 1.5 pounds of pollen per day. Brassica pollen is an excellent source of protein and contains all essential amino acids (Szcz sna, 2006). In a study of pollens from 16 different plant genera, crude protein in Brassica pollen averaged 24% (range in study: 13 to 25%), and total amino acids averaged 229 mg/g (range in study: 109 to 241 mg/g) (Szcz sna, 2006). Note: Brassica crops can be poisonous to mammalian livestock The same isothiocyanates that can inhibit weed seed germination and microorganism growth in soils can cause digestive problems in horses, cattle, sheep, swine, lambs and rabbits. Symptoms vary depending on the species but can include gastroenteritis, pain, salivation, diarrhea, upper GI tract disturbances and mouth irritation (“Poisonous Plants,” 2013). References: Antonious, G. F., M. Bomford, and P. Vincelli. 2009. Screening Brassica species for glucosinolate content. J. Envion. Sci. Health B. 44(3):311-6. Brassica. 2013. Wikipedia. http://en.wikipedia.org/wiki/Brassica Brown, P. D. and M. J. Morra. 2009. Brassicaceae tissues as inhibitors of nitrification in soil. J. Agric. Food Chem. 57:7706-7711. Cavanaugh, A. 2013. Brassicas: Cover Crops. extension.umass.edu/vegetable/articles/brassicas-covercrops. 4 Chapman, S. R. and L. P. Carter. 1976. Crop Production, Principles and Practices. W. H. Freeman and Company, San Francisco. 566 pp. Collins, S. A., J. K. Conner, and G. E. Robinson. 1997. Foraging behavior of honey bees (Hymenoptera: Apidae) on Brassica nigra and B. rapa grown under simulated ambient and enhanced UV-B radiation. Ann. Entomol. Soc. Am. 90:102-106. Free, 1993. Insect Pollination of Crops. Second Edition. Academic Press Limited, San Diego, CA. 684 pp. Hammer, O. 1966. Some problems of competition between summer rape and clover, in relation to pollination. In 2d International Symposium on Pollination, London, 1964. Bee World 47, supp. 1: 99-106. Kevan, P. G., H. Lee, and R. W. Shuel. 1991. Sugar ratios in nectars of varieties of canola (Brassica napus). J. Apicultural Res. 30(2):99-102. Larkin, R. and T. Griffin. 2003. Control of soilborne pathogens of potato with Brassica crop rotations. Phytopathology 93:S48 Masiunas, J. and C. Eastman. 2012. Glucosinolates in Brassicas: Biological control agents that are good for our health and bad for pests? Weed Control News. www.entomology.wisc.edu/mbcn/weed604.html. McGregor, S.E. 1976. Insect pollination of cultivated crop plants. Agriculture Handbook No. 496. USDA Agricultural Research Service. Mohammed, A. 1935. Pollination studies in tara (Brassica napus L. var. dichotsma Prain) and sarson (Brassica campestris L. var. sarson, Prain). Indian J. Agric. Sci. 5:125-154. Mohr, N. A. and S. C Jay. 1988. Nectar- and pollen-collecting behavior of honeybees on canola (Brassica campestris L. and Brassica napus L.). J. Apicultural Res. 27(2):131-136. Monfort, W. S., A. S. Csinos, J. Desaeger, K. Seebold, T. M. Webster, and J. C. Diaz-Perez. 2007. Evaluating Brassica species as an alternative control measure for root-knot nematode (M. incognita) in Georgia vegetable plasticulture. Crop Protection 26:1359-1368. Poisonous Plants, Genus: Brassica. 2013. Penn Veterinary Medicine Computer Aided Learning. http://research.vet.upenn.edu/PoisonousPlantsofPA/Brassica/tabid/5413/Default.aspx Szabo, T. I. 1982. Nectar secretion by 28 varieties and breeders’ lines of two species of rapeseed. Am. Bee J. 122:645-647. Szabo, T. I. 1985. Variability of flower, nectar, pollen and seed production in some Canadian Canola (Rapeseed) varieties. Am. Bee J. 124:341-342. Szcz sna, T. 2006. Protein content and amino acid composition of bee-collected pollen from selected botanical origins. J. Apicultural Sci. 50:81-90. 5