Comparative Assessment of Detergent-Based
advertisement

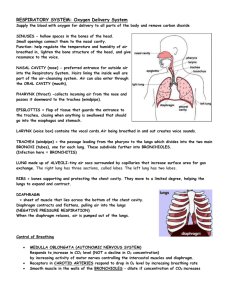
Alla Gent.ma Prof. ssa Maria del Zompo - Direttore del Dipartimento di Scienze Biomediche Universita’ degli Studi di Cagliari Cagliari, 3-6-2013 Resoconto delle ricerche svolte dal Dr. Roberto Loi presso il Weiss Laboratory, Vermont Lung Center, Department of Medicine, University of Vermont (USA) nel periodo di aspettativa per studio decorrente dal 1-42012 al 31-3-2013. Durante la sua permanenza presso il laboratorio diretto dal Prof. Daniel J. Weiss presso il Vermont Lung Center, il Dott. Loi si e’ occupato di studi concernenti l’utilizzo di cellule staminali mesenchimali e di cellule staminali pluripotenti indotte per il ripopolamento di “scaffolds” acellulari ottenuti mediante de-cellularizzazione di polmoni umani e murini. Gli studi condotti sono risultati nella pubblicazione di tre articoli su rivista per un Impact Factor medio di 7.5 e in due manoscritti ancora in preparazione. I risultati degli studi sono inoltre stati presentati a congressi internazionali. Elenco delle pubblicazioni derivanti dall’attivita’ di ricerca svolta dal Dr. Roberto presso il Vermont Lung Center – University of Vermont durante il periodo di aspettativa. Articoli su riviste 1) The effect of age and emphysematous and fibrotic injury on the recellularization of de-cellularized lungs. Sokocevic D, Bonenfant NR, Wagner DE, Borg ZD, Lathrop MJ, Lam YW, Deng B, Desarno MJ, Ashikaga T, Loi R, Hoffman AM, Weiss DJ. Biomaterials. 2013 Apr; 34(13):3256-69. 2) The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Bonenfant NR, Sokocevic D, Wagner DE, Borg ZD, Lathrop MJ, Lam YW, Deng B, Desarno MJ, Ashikaga T, Loi R, Weiss DJ. Biomaterials. 2013 Apr; 34(13):3231-45. 3) Endogenous Distal Airway Progenitor Cells, Lung Mechanics, and Disproportionate Lobar Growth following Long-Term Post- Pneumonectomy in Mice. Eisenhauer P, Earle B, Loi R, Sueblinvong V, Goodwin M, Allen GB, Lundblad L, Mazan MR, Hoffman AM, Weiss DJ. Stem Cells. 2013 (In press). Comunicazioni a congresso 1) Age and Injury Adversely Affect Re-Cellularization of De-Cellularized Lung. Sokocevic D, Bonenfant N, Wagner DE, Borg ZD, Lathrop M, Lam YW, Deng B, DeSarno M, Ashikaga T, Loi R, Hoffman AM, Weiss DJ. American Thoracic Society International Conference. Philadelphia (USA), May 17-22, 2013. 2) Cyclic mechanical stretch activates YAP/TAZ to regulate SP-C expression in distal lung epithelial cells. Wagner DE, Bonenfant NR, Borg Z, Sokocevic D, Loi R, Weiss DJ. American Thoracic Society International Conference. Philadelphia (USA), May 17-22, 2013. 3) Optimizing Lung De-Cellularization and Re-Cellularization: Effects of Storage and Sterilization. Bonenfant NR, Sokocevic D, Wagner DE, Borg ZD, Lathrop M, Lam YW, Deng B, DeSarno M, Ashikaga T, Loi R, Weiss DJ. American Thoracic Society International Conference. Philadelphia (USA), May 17-22, 2013. 4) Derivation of normal and cystic fibrosis human induced pluripotent stem cells (iPSCs) from airway epithelium. Loi R, Weiss DJ. 36th European Cystic Fibrosis Conference. Lisbon (Portugal) June 12-15, 2013. Sommario degli studi svolti dal Dott. Roberto Loi presso il Vermont Lung Center – University of Vermont durante il periodo di aspettativa. Abstract Use of stem cells for repopulation of de-cellularized cadaveric lungs scaffolds for ex vivo lung tissue generation offers a new potential therapeutic approach for clinical lung transplantation. Mesenchymal stromal cells (MSCs) obtained from bone marrow of adult mice can localize to lungs and acquire phenotypic and functional characteristics of differentiated lung epithelial cells, therefore they represent an option for re-cellularization of lung scaffolds. Notably, the remodeling of chromatin structure and the alteration of epigenetic marks that underlie lineage commitment, including histone methylation and acetylation and DNA methylation, can be induced by environmental cues, including by extracellular matrix (ECM)-dependent signaling. Optimal procedures for handling of de-cellularized lungs prior to the re-cellularization process have not been described to date. In fact, it is not yet clear how storage and sterilization of de-cellularized lungs affect the composition and properties of the resulting ECM, and the efficiency of the following re-cellularization process. Further, it is unclear how time of post-mortem storage of the lungs affects tissue characteristics and subsequent re-cellularization process, a relevant factor with procurement of human lungs from autopsy. To investigate this, we assessed the effects of advanced age, representative emphysematous and fibrotic injuries, and the combination of advanced age and emphysematous injury and found significant differences in both histologic appearance and in the retention of extracellular matrix (ECM) and other proteins, as assessed by immunohistochemistry and mass spectrometry, between the different conditions. Furthermore, we assessed effects of delayed necropsy, prolonged storage (3 and 6 months), and of two commonly utilized sterilization approaches: irradiation or final rinse with peracetic acid, on architecture and extracellular matrix (ECM) protein characteristics of de-cellularized mouse lungs. These different conditions resulted in significant differences in both histologic appearance and in retention of ECM and intracellular proteins as assessed by immunohistochemistry and mass spectrometry. Finally, we assessed binding and proliferation of bone marrow-derived mesenchymal stromal cells (MSCs) in de-cellularized scaffolds, using mouse epithelial C10 cells as a reference. Despite the observed differences among scaffold conditions, binding, retention and growth of bone marrow-derived mesenchymal stromal cells (MSCs) over a one month period following intratracheal inoculation was similar between experimental conditions. In contrast, significant differences occurred with C10 mouse lung epithelial cells. Therefore, age, lung injury, delayed necropsy, duration of scaffold storage, sterilization approach, and cell type used for re-cellularization may significantly impact the usefulness of this biological scaffold-based model of ex-vivo lung tissue regeneration. Introduction Bone marrow-derived Mesenchymal Stem Cells (MSCs) from adult mice can localize to recipient mouse lungs and acquire phenotypic characteristics of airway or alveolar epithelial cells [1,2]. These findings raise the possibility that abnormal lung epithelium can be repopulated with functional cells of bone marrow origin. As an alternative to in vivo repopulation of diseased lung epithelium, increasing interest in the use of de-cellularized complex whole organ scaffolds for ex vivo tissue engineering has provided both opportunity and also unique challenges. Ex vivo tissue engineering has been successfully used for the regeneration and clinical transplantation of tissues such as skin, cartilage, and bone [3]. Engineering organs with more structural and cellular complexity such as heart, lung, and liver is a more challenging endeavor, yet recent advances in tissue engineering techniques and in regenerative medicine have established a foundation upon which the functional replacement of these organs appears possible [3-4]. One promising approach involves the use of naturally occurring 3dimensional extracellular matrix (ECM) obtained by the de-cellularization of whole organs. The matrix serves as a biologic scaffold for ex vivo recellularization and generation of functional tissue with either differentiated adult cells or potentially by stem/progenitor cells [5]. The unresolved issues which still require clarification include defining optimal, organ specific approaches for de-cellularization and for sterilization and storage of de-cellularized organs prior to re-cellularization [3-6]. A number of recent publications have comparatively assessed different de-cellularization protocols for trachea and lung. Notably, architecture and extracellular matrix (ECM) protein composition of either trachea or lungs may differ substantially between different de-cellularization regimens [7-9]. Whether this difference will subsequently affect re-cellularization and generation of functional tissue remains to be clarified [6,7]. Methods of optimal sterilization and storage have been already developed for trachea [9,10] but not yet clearly established for decellularized lungs. One further consideration is that of post-mortem time prior to lung harvest and de-cellularization, a practical issue for procurement of human lungs. Several hours or even days may pass prior to post-mortem tissue harvest and it is still unknown whether this delay will affect the suitability of the donor lung for de-cellularization and subsequent re-cellularization. To address these questions, we assessed architecture and ECM protein content and distribution in mouse lungs obtained following a prolonged postmortem period prior to harvest compared to freshly procured lungs. We also assessed lungs obtained immediately after euthanasia and then subsequently stored after de-cellularization for prolonged periods (3 and 6 months). We further evaluated effects of sterilization using either irradiation or final rinse with peracetic acid, a commonly used protocol in storage of other biologic scaffolds [11-15]. We then assessed growth of two different cell types, murine bone marrow-derived mesenchymal stromal cells (MSCs) and C10 mouse lung type 2 alveolar epithelial cells, following intratracheal inoculation into the different decellularized lungs. Another relevant issue in the application of de-cellularized cadaveric lungs scaffolds for ex vivo lung tissue generation is represented by the fact that some of the donor lungs that might be utilized for de-cellularization and ex vivo bioengineering may originate from aged donors, donors with pre-existing structural lung diseases, or a combination of both age and lung disease. At present it is unknown how these factors might affect either de-cellularization or subsequent re-cellularization. Therefore, to assess these questions, we included in our analysis the comparative assessment of architecture and ECM content in de-cellularized mouse lungs from young (8-12 week) vs old (15-18 month) mice, lungs from young mice after induction of either emphysematous lung injury following intratracheal inoculation with elastase or of fibrotic injury following intratracheal instillation of bleomycin, or in young mice injured with elastase and allowed to age. Also in this case we assessed growth of murine bone marrowderived mesenchymal stromal cells (MSCs) in parallel with C10 mouse lung epithelial cells following intratracheal inoculation into the different de-cellularized lungs. Materials and Methods Mice Adult C57BL/6J male mice aged 8-12 wks (young mice) or 15-18 months (old mice), (Jackson Laboratories) were maintained at UVM in accordance with institutional and American Association for Accreditation of Laboratory Animal Care (AAALAC) standards and review. Lung Injury Emphysematous lung injury in mice was induced by oropharyngeal inoculation of porcine pancreatic elastase (USB) at a dose of 135 IU/mg (1.5 IU PPE) per kg body weight (Elastin Products). Mice were euthanized either one month later (young mice) or approximately 43-68 weeks later (old mice) and the heart-lung blocs subsequently de-cellularized. To induce fibrotic lung injury in mice, 0.075U/mouse of bleomycin (APP Pharmaceutical) was instilled by oropharyngeal inoculation. The heart-lung blocs were harvested 14 days postinstillation and subsequently de-cellularized. Lung De-cellularization Mice and rats were euthanized by lethal intraperitoneal injection of sodium pentobarbital. After opening the chest, the trachea was cannulated with a blunted Luer-lock syringe and the heart-lung bloc was harvested. The lungs underwent de-cellularization and were subsequently stored for certain periods of time or underwent specific sterilization techniques. The lungs were de-cellularized under sterile conditions according to previously published protocols [7,8,16-18]. Lungs were washed in de-ionized water (DI) containing 5X penicillin/streptomycin ( Cellgro) for one hour at 4°C. The lung was rinsed five times by injection of 3mL of the de-ionized water solution through the cannulated trachea. The vasculature was rinsed by injection of 15 cc total volume through the right ventricle. 3cc of 0.1% Triton X (Sigma) and 5X pen/strep in de-ionized water were then infused through both the trachea and the right ventricle, and the lungs were submerged in Triton X solution and incubated at 4° C for 24 hours. The following day, the lungs were rinsed with pen-strep solution as described above. 3cc of 2% sodium deoxycholate (Sigma) and 1X pen/strep in de-ionized water were then infused through the trachea and right ventricle and the lungs incubated in this solution at 4° C for 24 hours. The lungs were then rinsed with the de-ionized water as described above. 3cc of 1M NaCl and 5X pen/strep were then infused through the trachea and right ventricle and the lungs incubated in the solution for 1 hour at room temperature. The lungs were then removed from the NaCl solution and rinsed with de-ionized water as described above. 3cc of 30ug/mL porcine pancreatic DNase (Sigma), 1.3mM MgSO4 (Sigma), 2mM CaCl2 (Sigma), 5X Pen/Strep were then infused through the trachea and right ventricle and the lungs incubated in the solution for 1 hour at room temperature. Finally the lungs were removed from the DNase solution and rinsed with 5x pen/strep in 1x PBS as described above for the DI solution rinses. Lungs were stored in PBS/penstrep solution at 4° C until utilized. To assess effects of prolonged storage of the de-cellularized lungs, lungs were stored in sterile PBS with 5X penicillin/streptomycin at 4°C for either 3 or 6 months prior to analysis. To assess effects of two different sterilization approaches, one set of de-cellularized whole lungs was rinsed three times, through both the trachea and right ventricle, with 15mL of a 0.1% peracetic acid in 4% ethanol solution and then incubated in this solution for two hours prior to assessment [11,12]. Another set of de-cellularized whole lungs was irradiated for 12 minutes at a constant dose of 6 Gy/ minute using a RadSource 2000 Biological Irradiator prior to assessment [13-15]. To assess effects of delayed harvest, mice were euthanized and then kept at 4°C for 72 hours prior to necropsy and removal of the heart-lung bloc for subsequent de-cellularization and re-cellularization. Lung Histology De-cellularized lungs were fixed by gravity (20 cm H2O) with 4% paraformaldehyde for 10 minutes at room temperature, embedded in paraffin, and 5-µm sections mounted on glass slides. Following deparaffinization, sections were stained with hematoxylin & eosin, Verhoeff’s Van Gieson (EVG), Masson’s Trichrome, or Alcian Blue, and were assessed by standard light microscopy [7,16]. Immunohistochemical (IHC) Staining Deparaffinization was performed with three separate 10 min incubations of xylenes, followed by sequential descending ethanols and rehydration in water. Antigen retrieval was performed by heating tissue sections in 1x sodium citrate buffer (Dako) at 98⁰C for 20 minutes followed by a 15 min cooling step at room temperature. Tissue sections were permeabilized in 0.1% Triton-X solution (Sigma Aldrich) for 15 min. Triton-X was removed with two 10 min washes in 1% BSA solution. Blocking was performed with 10% goat serum for 60 min. After blocking, primary antibody was added and tissue sections were incubated overnight at 4°C in a humidified chamber. Tissue was washed three times with BSA solution for 5 min each. Secondary antibody was added and incubated for 60 min at room temp in a humidified chamber in the dark. Tissue was washed three times in BSA solution for 5 min each in the dark. DAPI nuclear stain was added for 5 min at room temperature in the dark followed by 2 washes in BSA solution for 5 min each. Tissue was submerged in Aqua Polymount (Lerner Laboratories), and a cover slip was added.. Slides were stored at 4°C in the dark to preserve fluorescence. Primary antibodies used were: Laminin antibody polyclonal (ab11575 – 1:100 – Abcam), Smooth muscle myosin heavy chain 2 polyclonal (ab53219 – 1:100 – abcam), Purified Mouse Anti-Fibronectin monoclonal (10/Fibronectin – 1:100 – BD Transduction Laboratories), Collagen I polyclonal (ab292 – 1:100 – abcam), Ki67 Proliferation marker polyclonal (ab16667 - 1:50 - abcam), Mouse clone anti-human Actin polyclonal (1A4 1:10,000 - Dako), Rabbit polyclonal to alpha elastin (ab21607 – 1:100 – abcam), Cleaved Caspase-3 polyclonal (Asp175 – 1:100 – Cell Signaling Technology). Secondary antibodies used: Alexa Fluor 568 goat anti-rabbit IgG (H+L) (1:500, Invitrogen), Alexa Fluor 568 F(ab’)₂ fragment of goat anti-mouse IgG (H+L) (1:500, Invitrogen) [7,16]. Mass Spectrometry Six samples (three duplicate pieces, of the same approximate volume and weight, were obtained from similar parenchymal regions of lungs de-cellularized with the Triton/SDC, SDS, and CHAPS protocols, respectively, and processed according to standard protocol [7, 16]. Each sample was loaded in triplicate onto a fused silica microcapillary LC column packed with C18 reversed-phase resin. Peptides were separated at a flow rate of 250 nL/min for 45 min. Nanospray ESI was used to introduce peptides into a linear ion trap quadrupole (LTQ) Orbitrap mass spectrometer (Thermo Electron). Mass spectrometry data were acquired in a data-dependent acquisition mode, in which a full orbitrap-MS scan (from m/z 400-2000, resolution r=30,000 at m/z 400) was followed by 10 LTQ-MS/MS scans of the most abundant ions. After an LC-MS run was completed and spectra obtained, the spectra were searched against the IPI Mouse protein sequence databases (V 3.75) using SEQUEST (Bioworks software, version 3.3.1; Thermo Electron, San Jose, CA), with search parameters detailed in Supplemental Methods. Proteins that were identified by two or more peptides in each of the six samples were regarded as identified. Proteins that were found at least in 2 out of 3 LC-MS/MS replicates were included in the analysis. Cells and Cell Inoculation Mesenchymal stromal cells (MSCs) derived from bone marrow of adult male C57BL/6 mice were obtained from the NCRR/NIH Center for Preparation and Distribution of Adult Stem Cells at Texas A and M University [19]. MSCs were cultured on cell-culture treated plastic at 37°C and 5% CO2 in MSC basal medium consisting of Iscove’s Modification of Dulbecco’s Medium supplemented with 2 mM L-glutamine, 100 U/ml penicillin and 100μg/ml streptomycin (Fisher), 10% fetal bovine serum (Atlanta Biologicals) and 10% horse serum (HS, Invitrogen). Cells were used at passage 9 or lower and maintained in culture at confluency no greater than 70%. Purity was determined by expression of Sca-1, CD106, CD29, absence of CD11b, CD11c, CD34, and CD45 expression, and the ability to differentiate into osteoblasts, chondrocytes and adipocytes in vitro [19]. C10 mouse lung type 2 alveolar epithelial cells were obtained courtesy of Matthew Poynter Ph.D., University of Vermont and cultured under standard conditions [20]. The right lobes were tied off using sterile suture under sterile conditions, and then then removed. 2x106 MSCs or C10 cells suspended in 1 mL MSC or C10 basal media, respectively, were mixed with 1ml of low-melting temperature agarose (Cambrex) and the 2mL cell suspension injected through the cannulated trachea into the left lung. The inoculated lung was then incubated for 30 minutes at 4°C until the agarose hardened and the lobe sliced with a sterile razor blade to yield transverse sections of approximately 1mm in thickness. Each slice was placed in a well of a sterile 24-well dish, covered with sterile cell media and placed in a standard tissue culture incubator at 37°C until agarose melted out of the tissue. The lungs were then submerged overnight in basal MSC or C10 media at 37°C and 5% CO2. The next day, medium was changed to either fresh basal medium [7]. Individual slices were harvested at 1, 3, 7, 14, 21, and 28 days post-inoculation, fixed for 10 minutes at room temperature in 4% paraformaldehyde, and mounted 5 μm paraffin sections were assessed by H and E staining for presence and distribution of the inoculated cells. Statistical Analyses Heat maps for the natural log of unique peptide hits for each positively identified protein in the mass spectrometric analyses of lungs de-cellularized under each experimental condition were generated using the 'pheatmap' package for 'R' statistical software version 2.15.1. Two group comparisons were done using the non-parametric exact permutation test with p<0.05 considered statistically significant [21]. Non-parametric Spearman correlations were also done with concordance considered significant at p <0.05 [21]. The exact permutation tests and correlations were done using SAS statistical software, version 9.2. Results Architecture and ECM composition of the decellularized mouse lungs Histologic evaluation with H&E, Verhoeff’s Van Gieson (EVG), and Masson’s trichrome stains demonstrates, as it’s been shown by us and others [7,8,1618,22-26], that freshly de-cellularized lungs, compared to native lung, maintain the architecture of the extracellular matrix. Glycosaminoglycans (GAGs) were less evident by Alcian Blue staining in freshly de-cellularized lungs, likely representing in large part loss of cell-associated GAGs during the decellularization process [7,16,18]. Overall, delayed necropsy appeared to minimally affect the histologic appearance and presence of collagens (Trichrome), elastin (EVG), and GAGs (Alcian Blue). Following 3 months of storage, scattered areas of atelectasis were observed particularly in central regions of the de-cellularized lungs. Following 6months storage, the de-cellularized lungs were markedly atelectatic, showing very different morphology compared to native or freshly de-cellularized lungs. Peracetic treated lungs had a similar appearance to native or freshly decellularized lungs although some central regions appeared more atelectatic. In contrast, irradiated lungs demonstrated an abnormal appearance with scattered heterogenous pattern of thickened alveolar septa, and large emphysematousappearing alveolar spaces. The lung architecture of de-cellularized lungs obtained from the 3 month storage, peracetic acid, and to some degree the irradiated lungs better resembled native or freshly de-cellularized lungs. In contrast, no significant improvement was observed in the 6 month storage lungs. As we and others have previously demonstrated, type 1 collagen and laminin were largely retained in freshly de-cellularized lungs whereas elastin was significantly decreased. Fibronectin was mostly retained but became fragmented-appearing [7,16]. As previously demonstrated, some cellular proteins, including smooth muscle actin & smooth muscle myosin were also retained in freshly de-cellularized lungs [7,16]. Neither delayed necropsy, 3 or 6 month storage, or peracetic acid treatment had any apparent effect on the presence of these proteins although the 6 month storage lungs remained abnormal appearing. In irradiated lungs, staining for laminin and collagen-1 appeared to be more intense, likely due to the clumping and thickened tissue. Fibronectin, smooth muscle actin, and smooth muscle myosin appeared to be present in the same patterns and intensities observed in native or freshly decellularized lungs. Residual protein composition of de-cellularized lung scaffolds Mass spectrometry was utilized to detect differences in residual protein content under the different storage and sterilization procedures. Freshly de-cellularized right lower lobes were used as controls. Proteins were assigned to one of five groups; ECM, cytoskeletal, intracellular cytosolic, intracellular nuclear, and membrane associated. Heat maps were generated with each positively identified protein and its corresponding number of unique peptide hits. The delayed necropsy lungs contained statistically significant increases in a large number of cellular associated proteins (non ECM) as compared to freshly de-cellularized lungs. Notably, several proteins associated with erythrocytes including hemoglobins A and B and the erythrocyte membrane protein Slc4a were markedly increased in the delayed necropsy as compared to fresh decellularized lungs. Peracetic acid-treated lungs contained a significantly higher number of ECM components compared to the controls. Lung scaffolds which had been stored in PBS for 3 months at 4ºC contained several ECM components, such as laminins, aggrecan, fibrillin, and myosins, which were significantly increased compared to freshly de-cellularized scaffolds. There were no ECM proteins which achieved statistically significant differences in scaffolds which had been stored for 6 months or between the 3mos vs 6mos or irradiated vs. acid-treated group comparisons. Mass spectrometry was also utilized for assessment of residual protein content and composition in decellularized lungs obtained from young, old, elastase, and bleomycin-injured mouse lungs We hypothesized that residual protein content would differ between young and old mice, and normal (young or old) vs. injured lungs. Lungs (right lower lobes) which had been freshly de-cellularized from young naïve mice were utilized as controls. Comparisons included old vs. young naïve mice, old elastase vs. young elastase-injured mice, old vs. old elastase-injured mice, and young naïve mice vs. young bleomycin-injured mice. Heat maps were generated with proteins broadly categorized as cytoskeletal, extracellular matrix (ECM), intracellular cytoplasmic, intranuclear and membrane proteins. The majority of statistically significant differences appeared between groups in the ECM proteins. As compared to controls, de-cellularized lungs from old mice, or either young or old elastase-treated mice, contained statistically fewer overall ECM proteins. Decellularized lungs from bleomycin-injured mice contained significantly more residual ECM proteins, with increases in residual collagen 6a, fibrillin, fibrinogen, and fibronectin. De-cellularized lungs from young elastase-treated mice contained more residual overall ECM proteins compared to those from old elastase treated mice. Similarly there were higher levels of residual ECM proteins in de-cellularized lungs from old vs old elastase-treated mice. While there were statistically significant differences in levels of residual proteins in the other categories (cytoskeletal, cytosolic, membrane, nuclear) between the experimental groups, there was no clear or obvious association between experimental group and residual protein content. Growth of MSCs and C10 cells in de-cellularized lungs To assess the impact of the different storage and sterilization procedures on the re-cellularization of lung scaffolds, two cell types were inoculated into separate de-cellularized lungs via an intratracheal route (1x106 of each cell type per lung) and engraftment and survival were assessed in lung slices at 1, 3, 7, 14, 21, and 28 days. Similar initial localization and distribution (day 1) of both C10s and MSCs throughout the lungs were observed with the different storage and sterilization conditions as that seen in freshly de-cellularized scaffolds. As previously observed [7,16,17], many of the MSCs that initially lodged in parenchymal lung regions developed a bipolar elongated appearance over time. Also as previously observed, C10 cells develop an elongated phenotype over time as they grow along alveolar septa [7]. The length of time in which the cells remained viable in the scaffolds was variable, dependent upon both the cell type and the treatment of the de-cellularized scaffold. In the 6-month storage condition, no viable cells were observed after 7 to 14 days in culture. In all other conditions, MSCs survived robustly through 28 days of culture. The cells were localized throughout the tissue and retained their characteristic bipolar elongated phenotype. We had previously found strong Ki67 staining and minimal caspase3 staining of MSCs at both 1 and 28 days when cells were inoculated into freshly de-cellularized lungs [7,16]. In the current studies, Ki67 staining demonstrated the presence of actively proliferating cells throughout the lung slices for the different storage and sterilization conditions both at day 1 and day 28 after inoculation. Minimal apoptosis was observed by caspase-3 staining at days 1 or 28 for any condition except the 6 month storage in which increased caspase-3 staining was observed at 7 and 14 days. Similarly, we had previously observed sustained growth and spreading of intratracheally inoculated C10 cells along alveolar walls following either 1 or 14-28 days in culture [7]. In parallel, robust Ki67 and minimal caspase-3 staining was observed [7]. In contrast, C10 cells inoculated into different sterilization and storage conditions were largely nonviable or absent at different times ranging between 7 and 14 days in culture. At the last viable time point for each condition, the C10 cells were largely localized on the periphery of the tissue or lining the major airways. Ki-67 and caspase-3 staining demonstrated active proliferation and minimal early apoptosis 1 day after seeding but significant increase in apoptosis at or before the last viable time point at which cells were observed for each storage and sterilization condition. To assess re-cellularization in the injured or aged de-cellularized lungs, 1x106 MSCs or C10 epithelial cells were separately inoculated and engraftment and survival were evaluated in lung slices cultured for 1, 3, 7, 14, 21, and 28 days. On day 1, both MSCs and C10s were observed to primarily engraft in alveolar spaces. After one day of culture, MSCs acquired a characteristic spindle-shaped phenotype, and could be found scattered throughout the different de-cellularized lungs. In contrast, while C10 cells could be found growing throughout the 28 day period in de-cellularized lungs obtained from young, old, and bleomycin-injured mice, elastase-injured lungs retained no viable cells past 14 days in lungs obtained from either young or old mice. To determine the proliferation and apoptosis rate for C10s and MSCs during the culture period, Ki67 and caspase-3 expression was assessed after one day of culture and at the last time point at which viable cells were observed for each condition. In the current study, robust Ki67 expression and minimal caspase-3 expression was observed in MSCs after one day of culture under each condition. Following 28 days in culture, less evident Ki67 expression but increased caspase-3 expression was observed, especially in de-cellularized lungs obtained from elastase or bleomycin-injured lungs. Discussion Use of de-cellularized whole lung scaffolds for ex vivo generation of functional lung tissue may provide in the future a viable option for clinical lung transplantation [6-8,16-18,22-27]. As already shown for other tissue types including skin, muscle, bladder, successful use of biologic scaffolds has already entered clinical practice [3-6]. However, the complex 3-dimensional structurefunctional biology of the lung makes this a more difficult task. A number of recent reports have evaluated lung de-cellularization, re-cellularization, and implantation in rodent and primate models [7,8,16-18,22-26]. While these reports show the viability of this approach, a number of unanswered questions remain. For example, there is no consensus on the optimal ways of producing clinically useful de-cellularized lungs, including the different detergent and physical approaches to be applied. Recent studies demonstrate significant differences between the structure and protein content and also the mechanical properties of decellularized lungs produced using different approaches [7,8,26]. However, whether these differences will significantly affect subsequent re-cellularization and also the potential immunogenicity of the de-cellularized scaffolds remains unclear. Recent data suggests that initial binding and short term growth of stromal and epithelial cells inoculated into mouse lungs de-cellularized using different detergent-based approaches is similar [7]. However, more data on longer term growth and also on growth of other cell types to be inoculated into the de-cellularized lungs, including vascular endothelial cells is necessary. Other practical issues need to be considered for use of de-cellularized lung scaffolds. Biologic scaffolds such as bone, cartilage, and skin can be stored for prolonged periods of time prior to use, particularly if treated with irradiation or final rinse in peracetic acid for sterilization [11-15]. However, it is yet to be clarified whether these approaches or long term storage will be applicable for decellularized whole lungs. In this respect, recent data demonstrates that significant tissue breakdown can occur in de-cellularized tracheas stored for up to one year [10]. To address this issue, we initially evaluated freshly de-cellularized lungs that were stored under refrigerated sterile conditions for up to 6 months. Most of the lungs remained sterile with only infrequent episodes of bacterial or fungal contamination. Histologic assessment of the stored lungs demonstrated development over time of lung atelectasis and loss of native architecture. These changes were partly reversible with inflation in lungs stored for 3 months, but became irreversible with inflation following 6 months storage. Therefore our results suggest that de-cellularized lungs should not be stored beyond 3 months. Irradiation, even at a dose under the one commonly generally recommended for biologic materials according to International Standard of Organizations (1525kGy) [13-15], produced significant distortion that was only partly responsive to subsequent lung re-inflation. Peracetic acid, a denaturing agent used both for sterilization and to rinse out residual detergents and other reagents utilized during tissue de-cellularization [11,12], had less effect on the resulting architecture. Between the different storage and sterilization procedures examined in our study there were significant differences in residual protein content, as assessed by mass spectrometry. Compared to freshly de-cellularized lungs, the most relevant differences were observed in the scaffolds following delayed necropsy and with use of peracetic acid sterilization of freshly de-cellularized lungs. The presence of proteins characteristic of erythrocytes together with other intracellular proteins in the delayed necropsy group suggests that autolysis of red blood cells and other cells present in the lungs occurred over time, despite cold storage, and that the proteins released from autolysed cells are not completely removed by the decellularization approach utilized. Additionally, peracetic acid can act as a protein denaturing agent and in this respect it is commonly utilized to solubilize ECM components for protein detection. Thus, as espected, freshly de-cellularized peracetic acid-treated lungs contained a significantly increased number of ECM components compared to non-treated lungs. The increase in ECM components is therefore most likely not indicative of an absolute increase in ECM components, but rather of an increased solubilization of ECM components which were then more readily detected using mass spectrometry. Similarly, scaffolds stored for 3 months had higher ECM protein levels than controls, but these increases were absent in the scaffolds stored in the same conditions for 6 months. Survival and proliferation of mesenchymal stem cells (MSCs) inoculated into the airways was comparable between the delayed necropsy, 3 month storage, peracetic acid, and irradiated de-cellularized lungs, suggesting that appropriate preservation of ECM necessary for binding and subsequent cell growth and proliferation were preserved. Markers of apoptosis were observed following MSC culture in de-cellularized lungs stored for either 3 or 6 months, suggesting that prolonged storage of de-cellularized lungs may not support sustained cell growth. Viability of a type 2 alveolar epithelial cell line (C10) was diminished time in all the experimental conditions tested compared to survival and proliferation of C10 cells inoculated into freshly de-cellularized lungs [7]. Markers of apoptosis were observed at early time points in culture, particularly in C10 cells seeded into freshly de-cellularized lungs stored for 3 or 6 months. These results suggest that commonly utilized approaches for storage and sterilization of other de-cellularized tissues and other types of biologic scaffolds may not be suitable for de-cellularized lungs. Another relevant issue for the potential applicability of de-cellularized lung scaffolds is represented by the fact that some cadaveric lungs may come from aged donors or from donors with previously existing structural lung diseases such as emphysema or pulmonary fibrosis. While advanced age or severe cases of either type of disease would not be suitable for consideration, moderately affected lungs could conceivably be utilized for de-cellularization and subsequent re-cellularization and clinical use. To evaluate this possibility, we assessed decellularization and initial re-cellularization of lungs obtained from aged mice and from mice with experimentally-induced emphysematous or fibrotic lung injury. The resulting ECM scaffolds for each condition were consistent with the underlying injury and also showed preservation of the characteristic injury patterns, as reflected by both histologic architecture and by proteomic assessment using mass spectrometry. This demonstrates that successful decellularization can be achieved in aged and injured lungs and that the resulting lung scaffold will reflect that original disease state. These findings are consistent with recent description of de-cellularized cadaveric lungs obtained from patients with idiopathic pulmonary fibrosis. Initial binding and subsequent survival and proliferation of a stromal cell line (MSCs) inoculated into the airways was robust across the different conditions and comparable to that observed following inoculation into de-cellularized lungs obtained from young healthy mice. The only exception was lack of initial cell engraftment and subsequent growth in the more densely fibrotic regions of the bleomycin-injured lungs, suggesting that appropriate preservation of the ECM structures necessary for initial binding and subsequent growth and proliferation were preserved. Similarly, engraftment and viability of an immortalized type 2 alveolar epithelial cell line (C10) was similar in aged and bleomycin injured lungs compared to that observed in freshly de-cellularized normal lungs. In contrast, despite good initial engraftment, survival of the C10 cells was diminished in emphysematous lungs produced by elastase treatment in both young and old mice. The reasons for this are not yet clear but one explanation could be that, despite preservation of ECM proteins in the elastase-injured lungs, more subtle and yet unidentified changes in the ECM scaffold do not support longer term proliferation and survival of cells. Notably, there was minimal residual elastin in young naïve de-cellularized lungs and no significant differences were detected between those compared to either young or old elastase-injured de-cellularized lungs. These results suggest that de-cellularized lungs obtained from aged lungs may be appropriate for ex vivo lung bioengineering approaches utilizing decellularization and re-cellularization strategies. Our data suggest that fibrotic lungs support prolonged growth of inoculated cells but whether these lungs will be useful for long-term regeneration yet needs to be determined. Recent data suggest that fibroblasts cultured in vitro on scaffolds consisting of pieces of decellularized lungs obtained from patients with idiopathic pulmonary fibrosis are induced to acquire a myofibroblast phenotype. This suggests that the specific scaffold obtained from de-cellularization of lungs from different disease states can significantly affect cell growth and differentiation. Accordingly, we found that de-cellularized emphysematous lungs may not support long term viability of epithelial cells. Our studies do not address the impact of the different condition tested on a wider range of cells including both mature pulmonary vascular endothelial cells as well as a range of stem and progenitor cells that might be utilized for recellularization. This will need to be done in a rigorous manner in future studies. References [1] Jiang, Y., et al., Pluripotency of mesenchymal stem cells derived from adult marrow. Nature, 2002. 418(6893): p. 41-9. [2] Krause, D.S., et al., Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell, 2001. 105(3): p. 369-77. [3] Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27-53. [4] Bhatia SK. Tissue engineering for clinical applications. Biotechnol J. 2010;5:1309-23. [5] Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1-13. [6] Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943-52. [7] Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB, et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods. 2012;18:420-32. [8] Jensen T, Roszell B, Zang F, Girard E, Matson A, Thrall R, et al. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng Part C Methods. 2012;18:632-46. [9] Haykal S, Soleas JP, Salna M, Hofer SO, Waddell TK. Evaluation of the structural integrity and extracellular matrix components of tracheal allografts following cyclical decellularization techniques: comparison of three protocols. Tissue Eng Part C Methods. 2012;18:614-23. [10] Baiguera S, Del Gaudio C, Jaus MO, Polizzi L, Gonfiotti A, Comin CE, et al. Long-term changes to in vitro preserved bioengineered human trachea and their implications for decellularized tissues. Biomaterials. 2012;33:3662-72. [11] Hodde JP, Record RD, Tullius RS, Badylak SF. Retention of endothelial cell adherence to porcine-derived extracellular matrix after disinfection and sterilization. Tissue Eng. 2002;8:225-34. [12] Sullivan DC, Mirmalek-Sani SH, Deegan DB, Baptista PM, Aboushwareb T, Atala A, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33:7756-64. [13] Freytes DO, Stoner RM, Badylak SF. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. J Biomed Mater Res B Appl Biomater. 2008;84:408-14. [14] ISO. Sterilization of health care products -- Radiation -- Part 1: Requirements for development, validation and routine control of a sterilization process for medical. ISO Technical Committee TC 1982006. [15] ISO. Sterilization of health care products – Radiation – Part 2: Establishing the sterilization dose. ISO Technical Committee TC 1982011. [16] Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, et al. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A. 2012;18:1-16. [17] Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, et al. A Nonhuman Primate Model of Lung Regeneration: DetergentMediated Decellularization and Initial In Vitro Recellularization with Mesenchymal Stem Cells. Tissue Eng Part A. 2012. [18] Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581-91. [19] Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530-41. [20] Malkinson AM, Dwyer-Nield LD, Rice PL, Dinsdale D. Mouse lung epithelial cell lines--tools for the study of differentiation and the neoplastic phenotype. Toxicology. 1997;123:53-100. [21] Zar JH. Biostatistical analysis. Englewood Cliffs, N.J.,: Prentice-Hall; 1974. [22] Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213-21. [23] Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565-80. [24] Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissueengineered lungs for in vivo implantation. Science. 2010;329:538-41. [25] Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92:998-1005; discussion -6. [26] Petersen TH, Calle EA, Colehour MB, Niklason LE. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs. 2012;195:22231. [27] Panoskaltsis-Mortari A, Weiss DJ. Breathing new life into lung transplantation therapy. Mol Ther. 2010;18:1581-3.