Pertwee_Info_for_AAAS_press
advertisement

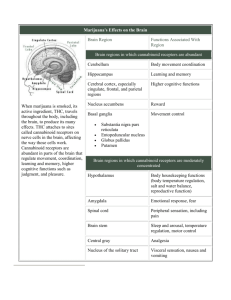
-1- The Pharmacology and Therapeutic Potential of Cannabinoids Roger Pertwee Summary of talk 1 Introduction The pharmacological properties of cannabis have been exploited for at least 5000 years. Cannabis was one of the first plants to be used by man: for religious ceremonies socially/recreationally as a medicine. In contrast, the constituents of cannabis started to be identified less than 200 years ago e.g.: cannabinol (CBN): isolated from cannabis in the late 1980’s, structure elucidated in the 1930’s, and first synthesized in the 1940’s; delta-9-tetrahydrocannabinol (delta-9-THC): structure elucidated (and first synthesized) in 1964 cannabidiol (CBD): structure elucidated in 1963, and first synthesized in 1964; delta-9-tetrahydrocannabivarin (delta-9-THCV): first detected in cannabis in 1970 by a group of Oxford University researchers that included me. The above 4 compounds are part of a family of at least 104 compounds that are produced by cannabis and are known collectively as “phytocannabinoids” (cannabis also contains at least 441 other types of compound). Following its identification in 1964, it soon became clear that delta-9-THC is the main psychoactive constituent of cannabis. This discovery prompted research directed at establishing precisely how THC can produce a cannabis-like “high” and that led eventually to the discovery of a new type of G protein-coupled receptor, that is highly expressed, for example in many parts of the brain, that is now known as the “cannabinoid CB1 receptor, and that is the receptor that THC activates to produce a “high”. The discovery of the CB1 receptor prompted a collaborative research project carried out in Israel and in my laboratory that led to the discovery in 1992, and thereafter, that human and animal tissues produce compounds that can activate the CB1 receptors; we called the first of these “endocannabinoids” anandamide (ananda is a Sankrit word that means eternal bliss or happiness). Several other endocannabinoids have since been discovered, as has a second (CB2) cannabinoid receptor. Whereas the CB1 receptor is expressed mainly by nerves both within and outside the central nervous system, the CB2 receptor is expressed mainly by cells of the immune system. Cannabinoid receptors together with the endocannabinoids that target these receptors and the processes that generate, transport or metabolize endocannabinoids is known as the “endocannabinoid system”. Importantly, it is now generally accepted that this system plays an “autoprotective” role in certain disorders in manner that reduces unwanted symptoms (e.g. pain) or the progression of certain disorders (e.g. multiple sclerosis). The discovery that the endocannabinoid system has an autoprotective role has revealed exciting potential new pharmacological strategies for the treatment of several disorders - a topic that I shall address very briefly at the end of this talk. References (1) Pertwee RG (2006). Cannabinoid pharmacology: the first 66 years. British Journal of Pharmacology 147: S163-S171. (2) Mechoulam R, Hanuš LO, Pertwee R, Howlett AC (2014). Early phytocannabinoid chemistry to endocannabinoids and beyond. Nature Reviews Neuroscience 15: 757-764. (3) Pertwee, R.G. (2014) Elevating endocannabinoid levels: pharmacological strategies and potential therapeutic applications. Proceedings of the Nutrition Society 73: 96-105. (4) Pertwee, R.G. (Editor) (2014) Handbook of Cannabis. Oxford University Press. < http://ukcatalogue.oup.com/product/9780199662685.do > -2- 2 Delta-9-Tetrahydrocannabinol (Delta-9-THC) Although initial delta-9-THC research focused mainly on the pharmacological basis of those of its effects that resemble the effects that are sought after by those who take cannabis as a recreational drug, evidence also emerged that delta-9-THC (like cannabis) has medical applications, and this led (1) to the licensing of this phytocannabinoid in the 1980’s as an anti-emetic medicine (for suppressing chemotherapy-induced nausea and vomiting) and as an appetite stimulating medicine (for AIDS patients) and (2) to the inclusion of delta-9THC and cannabidiol (in approximately equal amounts) in a cannabis-based medicine called Sativex, which was licenced in 2005, for the relief of multiple sclerosis and cancer pain, and in 2010, for the amelioration of multiple sclerosis spasticity. Some of the beneficial effects of delta-9-THC rely on its ability to activate cannabinoid CB 1 and/or CB2 receptors. It is noteworthy, however, that (1) at concentrations at which it produces such activation delta-9THC can interact with at least 9 other pharmacological targets (non-cannabinoid receptors, uptake processes and enzymes) and (2) it has a unique pharmacological “fingerprint” that differs from the pharmacological “fingerprints” of endocannabinoids or of at least some synthetic drugs that activate cannabinoid receptors. References (1) Crowther, S.M., Reynolds, L.A. and Tansey, E.M. (eds) (2010). The Medicalization of Cannabis. Witness Seminar Transcript. Volume 40. The Wellcome Trust Centre for the History of Medicine, at UCL: the transcript of a Witness Seminar that was held at the Wellcome Trust Centre in 2009 and can be downloaded as a pdf, at no cost, from <http://www.history.qmul.ac.uk/research/modbiomed/Publications/wit_vols/44870.pdf >. (2) Pertwee, R.G. and Cascio, M.G. (2014). Known Pharmacological actions of delta-9-tetrahydrocannabinol and of four other chemical constituents of cannabis that activate cannabinoid receptors. In: Handbook of Cannabis, Chapter 6, pp. 115-136. (ed. Pertwee, R.G.). Oxford University Press. 3 Phytocannabinoids Other Than Delta-9-Tetrahydrocannabinol (Delta-9-THC) 3.1 Introduction In addition to delta-9-THC, cannabis is a source of at least 103 other phytocannabinoids. The relative concentrations of phytocannabinoids can vary widely from cannabis plant to cannabis plant and not all known phytocannabinoids are present in all cannabis plants. Also, the concentrations of some phytocannabinoids in cannabis, including delta-9-THC, delta-9-tetrahydrocannabivarin and cannabidiol, are affected by heat. The pharmacological properties and effects on health of most phytocannabinoids have yet to be investigated. The remainder of this talk focuses on just some of the pharmacological actions and possible therapeutic uses of four phytocannabinoids: delta-9-tetrahydrocannabivarin (delta-9-THCV), cannabigerol (CBG), cannabidiol (CBD) and cannabidiolic acid (CBDA). These are actions and uses that have been discovered in my laboratory (in vitro findings) or in the USA, Canada, Italy or Spain by some of my collaborators (in vivo findings). 3.2 Delta-9-Tetrahydrocannabivarin (Delta-9-THCV) Delta-9-THCV was first discovered in 1970 in a UK licenced cannabis-based medicine, known as “Tincture of Cannabis” that was withdrawn as a medicine in the UK in the early 1970’s. Since its discovery, delta-9-THCV has been found (1) to activate CB2 receptors and to block CB1 receptors both in vitro and in vivo at low doses, and (2) to produce signs of CB1 receptor activation in vivo but not in vitro at high doses. Evidence for these actions will be presented in my talk as will examples of potential therapeutic uses for delta-9-THCV that would exploit the manner in which it targets cannabinoid CB1 and/or CB2 receptors at low doses. These potential uses include the treatment of nicotine dependence, liver damage, Parkinson’s disease and stroke. Effects of delta-9-THCV on signs of some of these disorders observed in in vivo animal models will be briefly mentioned in my talk. Delta-9-THCV has also been found to target the 5-HT1A receptor, a non-cannabinoid G protein-coupled serotonin receptor, with significant potency both in vitro and in vivo. This it does by enhancing the activation -3- of this receptor by its agonists. This discovery prompted research that led to the finding that delta-9-THCV can reduce some signs of schizophrenia in a rodent model of this disorder. References (1) Pertwee, R.G. and Cascio, M.G. (2014). Known Pharmacological actions of delta-9-tetrahydrocannabinol and of four other chemical constituents of cannabis that activate cannabinoid receptors. In: Handbook of Cannabis, Chapter 6, pp. 115-136. (ed. Pertwee, R.G.). Oxford University Press. (2) Cascio, M.G., Zamberletti, E., Marini, P., Parolaro, D. and Pertwee, R.G. (2014) The phytocannabinoid, Δ9-tetrahydrocannabivarin, can act through 5-HT1A receptors to produce anti-psychotic effects. Br. J. Pharmacol. Published on-line. 3.3 Cannabigerol (CBG) CBG has been found to display considerable potency in vitro at activating non-cannabinoid G protein-coupled receptors for adrenaline and noradrenaline (epinephrine and norepinephrine) that are known as α2adrenoceptors. This discovery prompted research that led to the finding that CBG can act through these receptors in vivo to reduce signs of pain relief in two rodent models of inflammatory pain. Other potential therapeutic uses for CBG that would exploit its ability to activate α2-adrenoceptors will be briefly mentioned in my talk. Some in vitro research has also revealed that, in contrast to delta-9-THCV, CBG can block 5-HT1A receptors. This discovery prompted additional research that led to the finding that CBG can also block 5-HT1A receptors in vivo. There is evidence that signs of schizophrenia can be reduced not only by activators but also by blockers of the 5-HT1A receptor. This prompted research that led to the discovery that CBG can indeed reduce some signs of schizophrenia in an in vivo rodent model of schizophrenia. However, whether any of these reductions resulted from CBG-induced blockade of 5-HT1A receptors has still to be investigated. Other potential therapeutic uses for CBG that exploit its ability to block 5-HT1A receptors will be briefly mentioned in my talk. References (1) Cascio, M.G. and Pertwee, R.G. (2014) Known pharmacological actions of nine non-psychotropic phytocannabinoids. In: Handbook of Cannabis, Chapter 7, pp. 137-156. (ed. Pertwee, R.G.). Oxford University Press. 3.4 Cannabidiol (CBD) and Cannabidiolic Acid (CBDA) It has been known for a while that some effects of CBD are mediated by 5-HT1A receptors, and both in vitro experiments, performed in my laboratory, and in vivo experiments, performed by my collaborators, have yielded data showing that it shares the ability of delta-9-THCV to enhance drug-induced activation of this serotonin receptor. It was active only over a narrow dose range, producing no detectable enhancement of 5HT1A receptor activation at doses above or below this narrow dose range. We extended this in vitro and in vivo research with CBD to CBDA which we found be effective at enhancing the activation of 5-HT1A receptors (a) over a much wider dose range than CBD and (b) at much lower doses than CBD. It is noteworthy that in the cannabis plant, CBDA serves as a precursor of CBD: it is converted to CBD when cannabis is heated. Potential therapeutic uses for CBD and CBDA that would exploit their ability to enhance the activation of 5HT1A receptors will be briefly mentioned in my talk. References (1) Cascio, M.G. and Pertwee, R.G. (2014) Known pharmacological actions of nine non-psychotropic phytocannabinoids. In: Handbook of Cannabis, Chapter 7, pp. 137-156. (ed. Pertwee, R.G.). Oxford University Press. -4- 4 Summary and Future Directions THCV, CBG, CBD and CBDA have pharmacological actions that have revealed potential new therapeutic uses for these four phytocannabinoids (see above). Some possible directions for future research with these four compounds will be briefly mentioned. Other important future directions for cannabinoid research will also be very briefly considered: research concerning (1) cannabis and its constituents, (2) endocannabinoids, or (3) synthetic cannabinoids, including (a) drugs that modulate effects of endogenously released endocannabinoids by increasing their levels or changing the strength with which they activate cannabinoid receptors (allosteric modulators that are currently under investigation in my laboratory), and (b) designer drugs such as spice or K2 that activate cannabinoid CB1 receptors more strongly/efficaciously than delta-9-THC/cannabis, and that are taken by some people to produce “legal highs”. http://ukcatalogue.oup.com/product/9780199662685.do