Density calculations Assignment
advertisement
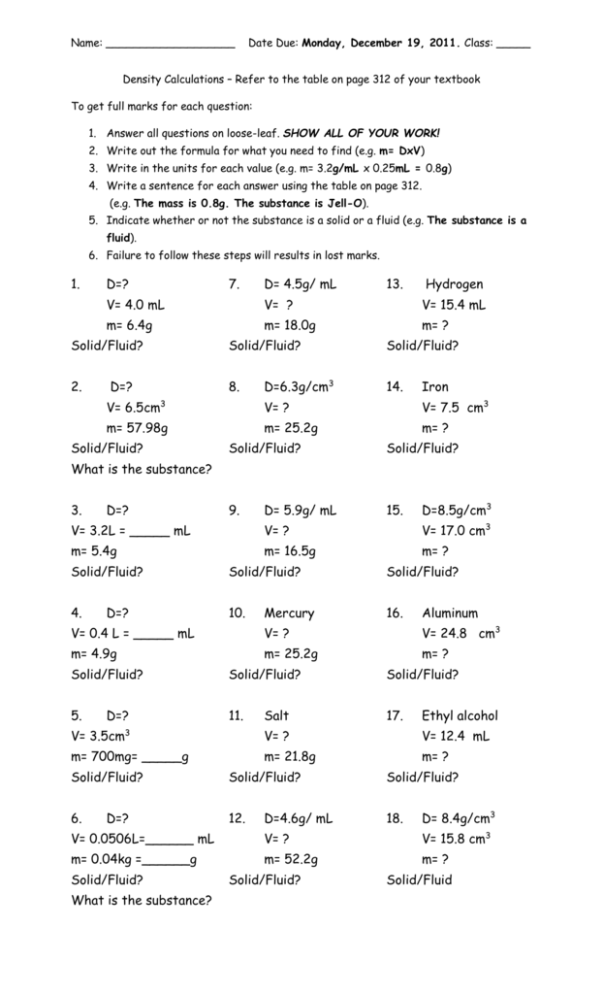
Name: ___________________ Date Due: Monday, December 19, 2011. Class: _____ Density Calculations – Refer to the table on page 312 of your textbook To get full marks for each question: 1. Answer all questions on loose-leaf. SHOW ALL OF YOUR WORK! 2. Write out the formula for what you need to find (e.g. m= DxV) 3. Write in the units for each value (e.g. m= 3.2g/mL x 0.25mL = 0.8g) 4. Write a sentence for each answer using the table on page 312. (e.g. The mass is 0.8g. The substance is Jell-O). 5. Indicate whether or not the substance is a solid or a fluid (e.g. The substance is a fluid). 6. Failure to follow these steps will results in lost marks. 1. D=? 7. D= 4.5g/ mL 13. Hydrogen V= 4.0 mL V= ? V= 15.4 mL m= 6.4g m= 18.0g m= ? Solid/Fluid? Solid/Fluid? 2. 8. D=? D=6.3g/cm3 Solid/Fluid? 14. Iron V= 6.5cm3 V= ? V= 7.5 cm3 m= 57.98g m= 25.2g m= ? Solid/Fluid? Solid/Fluid? Solid/Fluid? 9. 15. What is the substance? 3. D=? D= 5.9g/ mL D=8.5g/cm3 V= 3.2L = _____ mL V= ? V= 17.0 cm3 m= 5.4g m= 16.5g m= ? Solid/Fluid? Solid/Fluid? Solid/Fluid? 4. 10. 16. D=? Mercury Aluminum V= 0.4 L = _____ mL V= ? V= 24.8 cm3 m= 4.9g m= 25.2g m= ? Solid/Fluid? Solid/Fluid? Solid/Fluid? 5. 11. 17. D=? Salt Ethyl alcohol V= 3.5cm3 V= ? V= 12.4 mL m= 700mg= _____g m= 21.8g m= ? Solid/Fluid? Solid/Fluid? Solid/Fluid? 6. 12. 18. D=? D=4.6g/ mL D= 8.4g/cm3 V= 0.0506L=______ mL V= ? V= 15.8 cm3 m= 0.04kg =______g m= 52.2g m= ? Solid/Fluid? What is the substance? Solid/Fluid? Solid/Fluid Name: ___________________ Date Due: Monday, December 19, 2011. Class: _____





![Lymphatic problems in Noonan syndrome Q[...]](http://s3.studylib.net/store/data/006913457_1-60bd539d3597312e3d11abf0a582d069-300x300.png)

