1896 - Emerson Statistics
advertisement

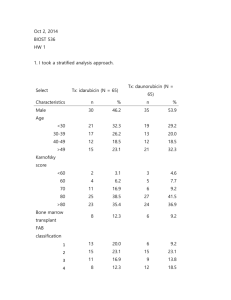
Homework code: 1896 1. Descriptive, univariate analysis was performed to determine characteristics of age, gender, laboratory results, FAB classification and Karnofsky score of the two treatment arms. To determine similarity between treatment arms, inferential testing was performed using chi-squared test for gender and unpaired t-test for other variables, see Table 1. Table 1: Characteristics of treatment arms Treatment type Age [range] Gender (%) WBC (SD) Platelets (SD) Hgb (SD) FAB class [95% CI] Idarubicin 38 [17-58] 30 male (46%) 29 (36.3) 66.6 (57.8) 9.2 (1.8) 2.95 [2.56-3.34] Karnofsky score [95% CI] 79.5 [76.7 – 82.4] Daunorubicin 39.8 [19-58] 35 male (54%) 43.3 (55) 93.6 (92.4) 9.6 (1.5) 3.19 [2.8 – 3.6] 79.5 [76.4 – 82.6] p-value 0.43 0.38 0.08 0.05 0.16 0.38 1.0 2. Using an intent to treat analysis, treatment arms were compared regarding the primary endpoint, which was induction of complete remission. Risk ratios were calculated for measure of association. Inferential testing was performed using chi-squared test. Among all 130 patients enrolled in the trial, the 75 patients who received idarubicin were 1.33 times more likely to experience induction of complete remission when compared to the 75 patients who received daunorubicin, 95% CI [1.05 – 1.7], p-value 0.014. 3. Decisions regarding adjustment for confounding should be made a priori based on scientific knowledge and plausibility. Gender frequently influences the effects of treatment and should be adjusted for if possible. 4. Logistic regression was performed to assess for any treatment benefit of idarubicin over daunorubicin while adjusting for gender. Inferential testing was based on likelihood ratio test statistic. After adjustment for gender, the 75 patients who received idarubicin had an odds of 2.5 of achieving complete remission when compared to the 75 patients who received daunorubicin, 95% CI [1.19-4.66], p-value 0.02. 5. To determine whether there was any treatment benefit for males receiving idarubicin, the treatment groups were stratified by gender. The frequency of primary endpoint of complete remission was compared between males who received idarubicin to those who received daunorubicin. Inference testing was performed using Pearson’s chi-squared test. Among the 30 males who received idarubicin, 21 (70%) achieved primary endpoint of induction of complete remission; in comparison, among the 35 males who received daunorubicin, 17 ( 48.5%) experienced complete remission, p-value 0.08. 6. Similar stratified analysis was performed to determine whether there was any difference in primary endpoint of complete remission among females who received idarubicin compared to daunorubicin. Among the 35 females who received idarubicin, 30 (85.7%) achieved primary endpoint of induction of complete remission; in comparison, among the 30 females who received daunorubicin, 21 (70%) experienced complete remission, p-value 0.12. 7. To determine the extent to which treatment benefit differs by sex, stratified risk ratios were calculated based upon gender classification. Inference testing was performed using chi-squared test. Mantel-Haenszel test for heterogeneity was used to determine if there was a significant difference of treatment benefit between gender groups. Stratified analysis revealed that the 35 women who received idarubicin were 1.22 times more likely to experience induction of complete remission when compared to the 30 women receiving daunorubicin, 95% CI [0.93-1.6]. In comparison, the 30 men who received idarubicin were 1.44 times more likely to experience induction of complete remission compared to the 35 men who received daunorubicin, 95% CI [0.95-2.18]. Though men appeared to have a greater treatment benefit with idarubicin, the difference in treatment benefit between gender groups did not achieve significance with inferential testing, p-value 0.5. 8. To account for any difference in treatment benefit by sex, a Mantel-Haenszel estimate was calculated as a common measure of association across the stratified categorical data. After adjustment for gender, the 75 trial participants who received idarubicin were 1.31 times as likely to experience primary endpoint of induction of complete remission as the 75 participants who received daunorubicin, 95% CI [1.04 – 1.66]. 9. Estimates of probability of complete remission were calculated for all combinations of treatment group and sex. Exact confidence intervals were calculated for logistic regression estimates, while Wilson-based confidence intervals were calculated for M-H estimates. Logistic Regression (MLE) Treatment Probability Estimate Female Male Idarubicin .867 [0.69 - .96] .714 [0.54 – 0.85] Daunorubicin .71 [0.54 – 0.85] .5 [0.31 – 0.69] OR [95% CI] 2.57 [0.73 – 9.1] 2.47 [0.86 – 7.11] Mental-Haenszel estimate (score) Treatment Probability Estimate Female Male Idarubicin .867 [0.7 – 0.94] .714 [.55 - .84] Daunorubicin .71 [0.55 – 0.84] .5 [.33 - .67] RR [95% CI] 1.22 [.93-1.6] 1.44 [.95 – 2.18] There are some slight differences between the treatment arms. The numbers of males and females differed, with 35 men and 30 women in the daunorubicin arm, and 35 women and 30 men in the idarubicin arm. The daunorubicin arm also had higher laboratory values, although this difference did not quite reach significance. Overall the groups appear well-matched at baseline. When evaluating estimates of probability of achieving complete remission, women in both treatment arms had a higher probability of achieving remission. In fact the probability of women in the daunorubicin arm achieving remission is similar to the probability of men in the idarubicin arm achieving remission. Overall among both genders, the idarubicin treatment arms had a higher probability of achieving remission. 10. It would be best to report the Mantel-Haenszel results, which are based upon score test. Score test will improve mean-variance relationship, which is an important consideration in categorical analysis of groups with relatively small sample sizes. In addition, when possible, it is best to report cohort or trial results in terms of relative risk or risk difference. Based on our stratified analysis, we can say that patients who receive idarubicin are 31% more likely to achieve complete remission that subjects who receive daunorubicin, 95% CI [4% - 66%]. .do file infile ptid str10 onstudy str1 tx str1 sex age fab karn wbc plt /// hgb str1 eval str1 cr crchemo str10 crdate str10 fudate /// str1 status str1 bmtx str10 bmtxdate str1 incl /// using http://www.emersonstatistics.com/datasets/leukemia.txt g onstJ= date(onstudy, "MD19Y") g fudtJ= date(fudate, "MD19Y") g obstime= fudtJ - onstJ g male= . replace male=1 if sex=="M" replace male=0 if sex=="F" g treat= . replace treat=1 if tx=="I" replace treat=0 if tx=="D" g eva= . replace eva=1 if eval=="Y" replace eva=0 if eval=="N" g creat= . replace creat=1 if cr=="Y" replace creat=0 if cr=="N" g outcome = . replace outcome=1 if status == "D" replace outcome=0 if status == "A" g transplant = . replace transplant=1 if bmtx == "Y" replace transplant=0 if bmtx == "N" **"Question 1" cs treat male ttest age, by(treat) ttest karn, by(treat) ttest fab, by(treat) **"Question 2" cs creat treat ** "Question 3" ** "Question 4" logistic creat treat male estimates store full quietly logistic creat treat lrtest full ** "Question 5" count if male == 1 & treat == 1 & creat == 1 count if male == 1 & treat == 1 & creat == 0 count if male == 1 & treat == 0 & creat == 1 count if male == 1 & treat == 0 & creat == 0 csi 21 17 9 18 ** "Question 6" count if male == 0 & treat == 1 & creat == 1 count if male == 0 & treat == 1 & creat == 0 count if male == 0 & treat == 0 & creat == 1 count if male == 0 & treat == 0 & creat == 0 csi 30 21 5 9 ** "Question 7 & 8" cs creat treat, by(male) ** "Question 9" cii 35 .7, binomial cii 30 .485, binomial cii 30 .857, binomial cii 35 .7, binomial cii 35 .7, binomial wilson cii 30 .485, binomial wilson cii 30 .857, binomial wilson cii 35 .7, binomial wilson