1721 - Emerson Statistics
advertisement

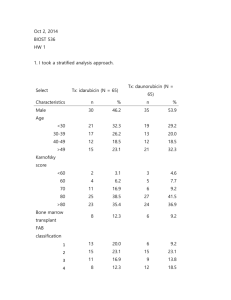
1721 HW1 1. Methods: Indicator variables were created for treatment arm (Daunorubicin and Idarubicin) and sex (male, female). Descriptive statistics are presented by treatment arm. Within each group defined by treatment arm, for continuous variables (age, Kamofsky score, baseline white blood cells, baseline platelets, baseline hemoglobin) the tables includes mean, standard deviation, minimum and maximum. For binary variable sex, percentages are presented. FAB Classification of AML subtype is an ordered categorical and we include mean, standard deviation, minimum and maximum as a comparison. Inference: Data is available on 130 subjects. See table for missing in each category. These subjects are omitted from all analyses. There are 65 subjects in each treatment arm and the table provides descriptive statistics within these two groups. Subjects in the Idarubicin arm were more likely to be young females. These subjects also had an overall slightly lower AML subtype compared to the Daunurubicin group. The baseline white blood cell count, platelet count, and hemoglobin were also lower compare to the Daunurubicin group. Table 1. Descriptive Statistics Male (%) Age (yrs) FAB Classification of AML Subtype (16)* Karnofsky score (0-100) Baseline white blood cells (103/mm3) ** Baseline platelets (103/mm3) *** Baseline hemoglobin (g/dl) **** Idarubicin n=65 46.20% 38 (12.47;17-61) Daunorubicin n=65 53.80% 39.8 (13.45;19-60) 2.95 (1.53;1-6) 3.2 (1.46;0-6) 79.5 (11.65; 30-100) 29.0 (36.32;0.4154.1) 66.6 (57.79;11-370) 9.2 (1.82;2.8-13.7) 79.5 (12.55;40-100) 43.3 (55.02: 0.7-215) 93.6 (92.39:11-457) 9.6 (1.49;6.4-13.9) *FAB has 8 missing values Daunorubicin 4 missing Idarubicin ** WBC has 1 missing value Daunorubicin *** Platelets 1 missing Daunorubicin **** Hemoglobin 1 missing Daunorubicin 2. Methods: The odds of subjects being in complete remission in the Idarubicin arm were compared to those in trial am D using a logistic regression model. Statistical inference was based on the wald statistic, with two-sided p-value and 95% confidence interval using the approximate normal distribution for logistic regression parameter estimates. This is a saturated model so robust standard errors are not needed. 1721 Inference: Of the 65 subjects randomized to the Idarubicin arm, the odds of complete remission from AML was 2.59 times those in the Daunorubicin arm. Based on a 95% confidence interval, this observed odds ratio would not be judged as unusual if the true odds ratio were anywhere between 1.20 and 5.59. A two-sided p-value of 0.016 suggests we can reject the null hypothesis that the odds of complete remission are not associated with treatment arm. 3. Inherently this randomized clinical trial should not be subject to confounding. However, with small samples sizes this can still occur. We can examine the association between sex and the exposure and outcome to assess potential confounding. Based on table 1 there are more females in the Idarubicin arm compared to the D arm. Additionally, when we examine the sex distribution among those in remission within each treatment arm we see that in the Daunorubicin arm 70% of women and only 49% of men are in remission. In the Idarubicin arm there is a similar pattern, 85% of women are in remission and 70% of men are in remission. Therefor sex is associated with both the treatment group and the outcome, even after randomization. If more women are likely to go into remission and there are more women in the Idarubicin arm then the results might be spuriously high for I treatment. 4. Methods: The odds of subjects being in complete remission in the Idarubicin arm were compared to those in the Daunorubicin using a logistic regression model. The variable sex was also included in the model to assess confounding. Statistical inference was based on the wald statistic, with two-sided p-value and 95% confidence interval using the approximate normal distribution for logistic regression parameter estimates. Robust standard errors were used. Inference: Of the 65 subjects randomized to the Idarubicin arm, the odds of complete remission from AML after adjusting for sex was 2.51 times those in the Daunorubicin arm. Based on a 95% confidence interval, this observed odds ratio would not be judged as unusual if the true odds ratio were anywhere between 1.14 and 5.53. A two-sided p-value of 0.022 suggests we can reject the null hypothesis that the odds of complete remission are not associated with treatment arm. When comparing these results to question 2, the model without sex, the odds ratio changes slightly indicating some small amount of confounding. 5. Methods: The odds of males being in complete remission in the Idarubicin arm were compared to males in the Daunorubicin arm using a logistic regression model. Statistical inference was based on the wald statistic, with two-sided p-value and 95% confidence interval using the approximate normal distribution for logistic regression parameter estimates. Inference: Of the 30 men randomized to the Idarubicin arm, the odds of complete remission from AML was 2.47 times men in the Daunorubicin arm. Based on a 95% confidence interval, this observed odds ratio would not be judged as unusual if the true odds ratio were anywhere between 0.89 and 6.88 A two-sided p-value of 0.084 suggests we fail to reject the null hypothesis that the odds of complete remission are not associated with treatment arm. 1721 6. Methods: The odds of females being in complete remission in the Idarubicin arm were compared to those in the Daunorubicin arm using a logistic regression model. Statistical inference was based on the wald statistic, with two-sided p-value and 95% confidence interval using the approximate normal distribution for logistic regression parameter estimates. Inference: Of the 35 women randomized to the Idarubicin arm, the odds of complete remission from AML was 2.57 times women in the Daunorubicin arm. Based on a 95% confidence interval, this observed odds ratio would not be judged as unusual if the true odds ratio were anywhere between 0.75 and 8.77 A two-sided p-value of 0.13 suggests we fail to reject the null hypothesis that the odds of complete remission are not associated with treatment arm. 7. Methods: An interaction term was created multiplying sex and treatment arm variables. A logistic regression model which included the variables for sex, treatment, and the interaction term was conducted. Statistical inference was based on the wald statistic, with two-sided p-value and 95% confidence interval using the approximate normal distribution for logistic regression parameter estimates. Robust standard errors were used. Inference: Based on the logistic regression model adjusting for sex and the sex-treatment interaction, the observed trend toward the OR measuring treatment effect is 3.92% lower in males than it is in females. The p-value of the interaction term (sex-treatment), p=0.961, indicates that we fail to reject the null hypothesis that treatment benefit of idarubicin compared to daunorubicin differs by sex. The 95% CI for the proportionate difference between the odds ratio measuring treatment effect in males that is 0.194 times are high or 4.75 times as high compared to the effect in females. 8. Methods: An interaction term was created multiplying sex and treatment arm variables. A logistic regression model which included the variables for sex, treatment, and the interaction term was conducted. Statistical inference was based on the wald statistic, with two-sided p-value and 95% confidence interval using the approximate normal distribution for logistic regression parameter estimates. Robust standard errors were used. Post-estimation command, testparm, was used after running the logistic model that included both the treatment and treatment-sex variables. Using a wald test, this tested the hypothesis that both regression coefficients were equal to zero. Inference: Based on the logistic model adjusting for sex and the sex-treatment interaction, and then using post-estimation commands, a p-value of 0.073 indicates we fail to reject the null hypothesis that idarubicin use is not associated with more frequent remission compared daunorubicin. 1721 9. Methods: Observed probabilities for each group were calculated by determining the number of subjects in remission by sex and treatment arm. The probabilities of remission for models 4 and 7 were calculated by exponentiating the sum of log odds of each parameter. The odds were then converted into probabilities. Inference: Moedls 4 and 7 are both similar to the observed probabilities for each group. The models are slightly different because they are borrowing information across groups in the model. Observed Female – Idarubicin Female - Daunorubicin Male – Idarubicin Male - Daunorubicin 30/35 (.86) 21/30 (.70) 21/30 (.70) 17/35 (.49) Model 4 (sex adjustment) 0.855423 0.702006 0.702006 0.483995 Model 7 (sex-treatment interaction) 0.857143 0.700000 0.700000 0.485714 10. I would choose the analysis in question 4 which uses a logistic regression model that adjusts for sex. We had some idea before analyzing the data that sex would confound the relationship (see question 3) and while this is an RCT it is quite small and there some confounding that was not accounted for in the randomization. It was never an a priori hypothesis that sex modifies the relationship between treatment and remission. We also do not have sufficient power to test this relationship. Therefore, showing results stratified by sex or using a model with an interaction term is not correct.