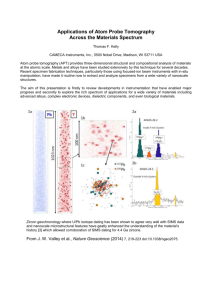
bit25152-sm-0001-SuppFig-S1
advertisement

Supplementary Methods Animals Primary hepatocytes were isolated from 6-8 week old adult male C57/Bl6 mice, weighing 20-25g (Jackson Laboratories, Bar Harbor, ME). Animals were housed two per cage within an isolated cubicle in a12h light/12h dark cycle animal room and allowed to access water and food ad libitum. To minimize diurnal variation, all procedures were initiated between 10:00am and 11:00am. Special care was taken to reduce animal stress by minimizing personnel and animal access to the room. All procedures were approved by the Massachusetts Institute of Technology Committee for Animal Care. PreSens Oxygen Concentration Measurements Dissolved oxygen was measured using 24-well OxoDish plates in conjunction with the SensorDish Reader from PreSens Precision Sensing GmbH. The OxoDish plates feature an immobilized fluorescent sensor spot 3mm in diameter at the center of each well of the culture plate. The plates are calibrated and sterilized by the manufacturer. Each well was coated with 9ug/cm2 rat tail collagen I before hepatocytes were plated and cultured as described above. The SensorDish Reader recorded real-time oxygen measurements every 3 minutes for each well in each experiment. All conditions were tested in triplicate from at 2 independent isolations. Calculating cell surface oxygen concentration from probe measurements. In order to assess the magnitude of the probe geometry on prediction of the oxygen gradient, and on the values of oxygen tensions for cells located directly beneath the probe, a 2-D discrete diffusion-reaction equation was used to model the oxygen concentration in cylindrical coordinates for a cross-section of a well with radius r and medium height h. An arbitrary initial condition is used and the model iterates forward in time until steady-state is reached. The probe was simulated at r=0 (i.e. precisely centered in the well) with various placement heights, medium depths, and cell consumption rates. For each condition simulated, two key outcomes were tabulated: (i) the offset between the probe measurement and the oxygen concentration at equivalent height (0.5mm above the cell surface) in an unaffected region of the dish where the profile is linear and (ii) the maximum deviation of the oxygen concentration in the hepatocellular microenvironment for cells located directly beneath the probe from the concentration in the environment of cells distant from the probe. A typical result illustrating key features of the model predictions is shown in Figure 2, in which the estimated oxygen concentration is represented in colorimetric form for a radial cross-section of the well, from the center of the well (r=0mm) to an unaffected area of the well (r=4mm). The probe is located in the center of the dish (r=0 to 0.25mm) at a distance of 0.5mm above the cells. The distortion of the oxygen concentration profile by the probe is most pronounced right at the probe tip, and it is at this vertical location that the difference between the oxygen concentration at center (r=0mm) and the bulk (r>1mm) is demonstrated by the shift from blue to green; in the example shown, this difference is ~8uM. The probe reports the bulk average of the concentration profile shown from r=0 to r=0.25 at the height of 0.5mm In experiments, the measured oxygen concentration at the 0.5mm probe depth was used with corrections from the simulations to predict the oxygen concentrations at the cell surface in the region under the probe and the bulk outside the corona of influence of the probe. The oxygen concentrations at the cell surface for the region beneath the probe were more appropriate for calculation of the dependence of oxygen consumption rate on local oxygen tension, whereas the concentrations at the cell surface outside the region beneath the probe – representing >99% of the total cell area – were relevant for the correlation with albumin secretion and other metabolic functions. Cell Viability Assay for Quantitative Imaging Analysis Cell viability assays were performed using live/dead viability kit (Invitrogen) to fluorescently label dead cells with ethidium homodimer-1. Hoechst was added to label all cell nuclei. Plates were imaged using a Compucyte Laser Scanning Cytometer and analyzed using Cell Profiler software. A 5mm2 area was recorded from each sample well using a 20x objective. Cell number was calculated by counting the numbers of cell nuclei using the Hoechst fluorescent images. Cells positively stained by ethidium homodimer-1 were recorded as dead cells. From this data, both cell viability and cell density were calculated for 2 timepoints, 24 hours and 4 days after isolation. Measurement of Albumin Medium was changed daily and samples collected and stored at -20ºC. Albumin was quantified from the collected media samples using a mouse albumin enzyme-linked immunosorbent assay (ELISA, Bethyl Laboratories) following the manufacturer’s protocol. Supplementary Figures Figure S1. Representative oxygen trace from hepatocytes cultured at 10% O2, 1.3mm medium depth. Probe measurements were collected every 3 minutes over the course of 7 days. 200 180 160 140 2 [O ] (uM) 120 100 80 60 40 20 0 0 1 2 3 4 time (days) 5 6 7