Unit 1 Chemistry
advertisement

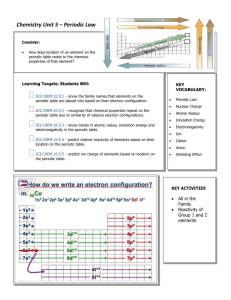
Mon Sep 1 Tue Sep 2 Wed Sep 3 Chemistry Unit 1 Density observations PPT lab Safety Brain Pop Lab Safety Plan and implement comparative & descriptive investigations by making observations & asking welldefined questions Mon Sep 8 Con’t: Students will explore structures of atoms, describe properties of matter, construct atomic models and explain the arrangement of elements found on the periodic table Mon Sep 15 Tue Sep Wed Sep 10 Thur Sep 4 Fri Sep 5 PPT presentation Students will with observation explore and inference structures of scenarios atoms, describe Lab Safety properties of matter, construct atomic models Quiz on Lab and explain the Safety arrangement of elements found on the periodic table Thur Sep 11 Fri Sep 12 Calculate number Calculate number Into to Periodic Into to Periodic of electrons, of electrons, Table Table protons & protons & PPT on Periodic PPT on Periodic neutrons neutrons table table Atomic mass vs. Atomic mass vs. atomic # Brain Pop clip PPT on Atomic Theory Tue Sep 16 atomic # Brain Pop clip PPT on Atomic Theory Wed Sep 17 Thur Sep 18 Fri Sep 19 Cont. Periodic Cont. Periodic Brain Pop Brain Pop Ionic, Covalent Table Table and Metallic Chapter 3 Chapter 3 bonding PPT on Periodic PPT on Periodic Assign Element Assign Element table table Finish Ch 3 Project Project Mon Sep 22 Ionic, Covalent and Metallic bonding Finish Ch 3 Mon Sep 29 Review for TEST Tue Sep LAB-Start Science Fair Go over scientific method and write-up procedures Tue Sep 30 Review for TEST Wed Sep 24 LAB-Start Science Fair Go over scientific method and write-up procedures Wed Oct 1 TEST Thur Sep Chemical Bonding Quiz Video of bonding Thur Oct 2 TEST Fri Sep 26 Chemical Bonding Quiz Video of bonding Fri Oct 3 HOLIDAY TEKS: SCI.8.1A Demonstrate safe practices during laboratory and field investigations as outlined in the Texas Safety Standards. SCI.8.2A Plan and implement comparative and descriptive investigations by making observations, asking well-defined questions, and using appropriate equipment and technology. SCI.8.2C Collect and record data using the International System of Units (SI) and qualitative means such as labeled drawings, writing, and graphic organizers. SCI.8.2E Analyze data to formulate reasonable explanations, communicate valid conclusions supported by the data, and predict trends. SCI.8.3A In all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning and experimental and observational testing, including examining all sides of the scientific evidence of those scientific explanations so as to encourage critical thinking by the student. SCI.8.3B Use models to represent aspects of the natural world such as human body systems, and plant and animal cells. SCI.8.3C Identify advantages and limitations of models such as size, scale, properties, and materials. SCI.8.3D Relate the impact of research on scientific thought and society, including the history of science and contributions of scientists as related to the content. SCI.8.4A Use appropriate tools to collect, record, and analyze information as needed to teach the curriculum. Ⓡ SCI.8.5A Describe the structure of atoms including the masses, electrical charges and locations of protons and neutrons in the nucleus and electrons in the electron cloud. Ⓡ SCI.8.5B Identify that protons determine an element’s identity, and valence electrons determine its chemical properties including reactivity. Ⓡ SCI.8.5C Interpret the arrangement of the Periodic Table including groups and periods, to explain how properties are used to classify elements.