Supplementary Notes 4
advertisement

SM286 – Spring 2010
Supplementary Notes 4
Particle in a Box (1D)
Particle in a Box (1 Dimension)
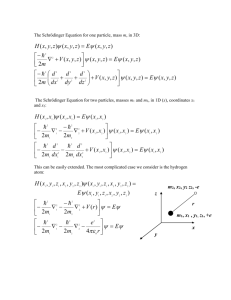
The time independent Schrödinger equation for a particle equation moving in one dimension:
−
ℏ2 𝑑2 𝜓(𝑥)
+ 𝑉(𝑥)𝜓(𝑥) = 𝐸𝜓(𝑥)
2𝑚 𝑑𝑥 2
Where:
ℏ
ℎ
2𝜋
(reduced Plank’s constant)
Plank’s constant (describes size of quanta in quantum mechanics)
ℎ
mass of particle
𝑚
wave function (replaces the concept of trajectory in classical mechanics)
ψ
𝑉(𝑥) potential energy of particle
total energy of particle
𝐸
For a particle in a one-dimensional box of length 𝐿 ,the potential
energy function is
0
𝑉(𝑥) = {
∞
0<𝑥<𝐿
.
elsewhere
This implies that the particle can only exist inside the box where
𝑉(𝑥) = 0.
Therefore:
−
ℏ2 𝑑2 ψ
𝑑2 ψ 2𝑚𝐸
=
𝐸ψ
→
+ 2 ψ=0
2𝑚 𝑑𝑥 2
𝑑𝑥 2
ℏ
.
Let:
𝑘2 =
2𝑚𝐸
𝑑2 ψ
→
+ 𝑘 2ψ = 0
ℏ2
𝑑𝑥 2
We have now reduced that equation to a homogeneous second order differential equation with
constant coefficients. We have shown earlier that the general solution to this equation is:
ψ(x) = c1 sin(kx) + c2 cos(kx)
The infinite potential outside of the box implies the following boundary conditions:
ψ(0)=0 and ψ(L)=0
1
SM286 – Spring 2010
Supplementary Notes 4
Particle in a Box (1D)
Furthermore, we can consider that ψ=0 for any point outside the box. Applying the first boundary
condition:
ψ(0) = c1 sin(0) + c2 cos(0) = 0 →
c2 = 0 →
ψ(x) = c1 sin(kx)
Applying the second boundary condition:
c1 sin(kL) = 0 → 𝑘𝐿 = 𝑛𝜋 → 𝑘 =
𝑛𝜋
𝐿
where n is an integer. Therefore:
nπx
ψn (x) = c1 sin (
)
L
Note that the subscript on ψ indicates that there are different solutions for different values of n. We
now need to determine c1 . Recall that:
2
L
L
|ψn (x)| = ∫ ψn (x)2 dx = ∫ c12 sin2 (
0
0
L
nπx
nπx
) dx = c12 ∫ sin2 (
) dx
L
L
0
2
Since |ψn (x)| represents the probability distribution function and we know that the particle will be
2
somewhere in the box, we know that |ψn (x)| =1 for 0 < 𝑥 < 𝐿, i.e. there is a 100% probability that the
particle is somewhere inside the box. Therefore:
L
nπx
c12 ∫ sin2 (
) dx = 1
L
0
We can show that:
L
nπx
1 sin(nπ) cos(nπ)
∫ sin2 (
) dx = L ( −
)
L
2
2nπ
0
This is the solution as it appears on the TI Voyage 200, but since n is an integer, sin(nπ) = 0. Hence:
L
nπx
L
∫ sin2 (
) dx =
L
2
0
and:
c12 L
2
= 1 → c1 = √
2
L
thus:
2
SM286 – Spring 2010
Supplementary Notes 4
Particle in a Box (1D)
2
nπx
ψn (x) = √ sin (
)
L
L
This is the solution to the wave equation for the particle in a one dimensional box.
We now turn our attention to the total energy. Recall:
𝑘2 =
2𝑚𝐸
ℏ2
Since:
𝑘=
𝑛𝜋
𝐿
ℎ
and ℏ = 2𝜋
We get:
𝐸=
ℏ2 𝑘 2
ℎ2 𝑛2 𝜋 2 1
𝑛 2 ℎ2
= 2 2
→ 𝐸=
2𝑚
4𝜋 𝐿 2𝑚
8𝑚𝐿2
Thus the energy is quantized (since n=1,2,3, … and all other terms are constant). The wave functions
and probability functions are plotted below for a box with length 𝐿 = 1 for corresponding energy levels.
Note that the plots have been shifted up by 𝑛2 for display purposes.
3
SM286 – Spring 2010
Supplementary Notes 4
Particle in a Box (1D)
With regards to the wave functions, we define a node as a location other that the endpoint where
ψn (x) = 0. Note that there are 𝑛 − 1 nodes that correspond to each energy level. Consider 𝑛 = 2,
there is one node. If we consider the probability distribution function for 𝑛 = 2, we see that it equals 0
at 1/2. If this is the case, how can a particle get from the left-half to the right-half of the box? You will
discuss a phenomenon called “tunneling” in subsequent chemistry classes to explain this behavior.
Example:
What is the probability that a particle in the ground state will be found between L/2 and 2L/3? (note:
ground state means 𝑛 = 1)
2
2L/3
∫
L/2
2
2L/3
|ψn (x)| dx = ∫
L/2
2
πx
2 2L/3
πx 2
√
(sin ( )) dx = . 3044 or 30.44%
| sin ( )| dx = ∫
L
L
L L/2
L
4
SM286 – Spring 2010
Supplementary Notes 4
Particle in a Box (1D)
Homework
1. For the potential well describes in these notes, what is the probability that a particle in the 2nd
energy level will be found between L/2 and 2L/3.
2. Assume that for the particle-in-box described in these notes that the potential energy inside the box
V(x)=1. Assume that the box goes from x=0 to x=2L. Find ψ(x). Find an expression for E in terms of
n, h, m, and L.
3. In problem 2, what is the probability that a particle in the 3rd energy level will be found between L/2
and 2L/3.
5