Biology - University of St. Thomas
advertisement

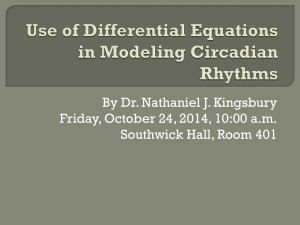
SABBATICAL LEAVE APPLICATION- BIOLOGY University of St. Thomas Title of project: Role of chromosome compaction in the cyanobacterial response to photoperiod. Abstract of project (150 word max.; 12 point Times New Roman font, single-spaced text) The cyanobacterium Synechococcus elongatus has circadian (daily) rhythms in behavior that are susceptible to light and temperature cues; allowing this organism to shift behavioral activities based upon environmental signals. The mechanism of the circadian clock that controls these behaviors in S. elongatus has been studied intensively. However, the portion of the clock that has received the least attention thus far, in any circadian model system, has been the mechanism by which the circadian clock communicates with the environment to interpret changes in day length (photoperiod), such that behavior can be appropriately adjusted. The work proposed here will investigate whether chromosome compaction plays a role in the ability of these organisms to adjust their behavior based on day length. This sabbatical will 1) forward my research agenda, 2) increase our collective understanding of the circadian mechanism, and 3) bring new research opportunities to UST students. NARRATIVE: a) Significance of this work: Cyanobacteria are found in nearly every habitat including most dry land ecosystems, geothermal hot springs, caves, and polar and hot deserts (Cox et al., 1981; Priscu et al., 1998; Brasier et al. 2002). The cyanobacterium Synechococcus elongatus is photoautotrophic, meaning it produces its own energy directly from sunlight and obtains all its carbon mass by fixing carbon dioxide, using photosystems similar to photosynthetic plants. Although S. elongatus is a unicellular organism, it performs a number of complex metabolic processes that take place at certain times of day. To efficiently regulate their cellular activities in a temporal fashion, cyanobacteria make use of a circadian clock (Ditty et al., 2009). The primary function of a circadian clock is to keep time on a 24-hour scale, which provides an internal estimate of time of day based on cues from the external environment (Pittendrigh, 1981; Johnson et al., 2003). The circadian clock allows an organism to be synchronized to the external environment such that it allows the organism to best fit their cellular 1 functions to the environment (e.g. photosynthesis during the day (Class 1) when light is available and purine biosynthesis at night (Class 2) (Ditty et al., 2009)). input oscillator output °C or LdpA CikA Pex KaiA KaiB KaiC SasA RpaA LabA chromosome compaction Figure 1. Schematic of the circadian clock in cyanobacteria (from Ditty et al., 2009) Synechococcus elongatus has circadian rhythms that are susceptible to light and temperature cues and can therefore shift behaviors by phase resetting, or changing when activity is at its peak based upon environmental signals. This process is exactly the same as to how humans shift behavior in response to travel across time zones. The genes responsible for the circadian clock, which control the circadian rhythms in S. elongatus, have been separated into three categories: oscillator genes, input genes, and output genes (Figure 1). The oscillator genes are kaiA, kaiB, and kaiC, named after the Japanese word kaiten meaning cycle or “turning of the heavens” (Ditty et al., 2003). The mechanism of the circadian clock in S. elongatus has been studied intensively. However, the portion of the clock that has received the least attention in this field thus far has been the mechanism by which the circadian clock communicates with the environment through input pathways. In particular, very little is known about how the circadian clock in cyanobacteria responds to photoperiod, or the duration of light versus the duration of darkness within a 24-hour day. One of the current problems in the cyanobacterial circadian rhythm field is that the vast majority of what we know about the circadian mechanism in S. elongatus has been based solely on photoperiods that consist of 12-hours of light and 12-hours of dark (12L:12D). Although different photoperiods have considerable impacts on the circadian activity 2 of organisms in other model systems (Dunlap et al., 2004; Johnson et al., 2003; Pittendrigh, 1981), we know nothing of how any organism responds to photoperiod at the molecular level. Therefore, the work proposed here is of major importance to the field of circadian biology in that it will be the first significant work to address this question. In addition, this work will expand my own research program and foster collaboration with the lab of Dr. Stanly Williams that has the expertise to unveil the molecular basis of photoperiod. b) Basic description of the proposed work: Recent work from the Williams lab has begun to shed light on the molecular mechanism of how the circadian clock conveys environmental information to gene expression. Their work has demonstrated that changes in chromosome compaction (defined as the physical accessibility of the chromosome for gene expression) are observed as a function of circadian time. It was found that the chromosome is relatively diffuse during the day and compacted at night. Therefore, it is thought that to generate circadian gene expression, the Kai proteins may communicate temporal information through chromosome compaction, which then controls access to gene expression based upon that compaction at certain times of day (Smith and Williams, 2006). The research proposed here will begin to investigate if chromosome compaction is a probable mechanism for mediating the effects of photoperiod on gene expression. Figure 2. Chromosome compaction of wild-type S. elongatus under 12L:12D photoperiod. Green; DAPI stained chromosomal DNA, Red; autofluoresence of cell membranes (from Smith and Williams, 2006). 3 Prior research has shown that chromosome compaction is a rhythmic process during a twenty-four hour period. It was found that in a 12L:12D photoperiod cycle, the chromosome goes through compaction for the first 12 hours of light and then decompaction of the chromosome for the second 12 hours of dark (Figure 2). Again, all of this research was preformed using a standard 12L:12D photoperiod, and nothing is known about the relationship between chromosome compaction and the cellular response to other photoperiods. Therefore, this project will investigate how chromosome compaction and phase response of the wild-type S. elongatus strain is affected in varying photoperiods. The methodology for this investigation is rather simple and will follow protocols published by the Williams lab (Smith and Williams, 2006). Briefly, wild-type S. elongatus cultures will be grown under exposure to different photoperiods; 12L:12D as a control, and 6L:18D, 8L:16D, 16L:8D, and 18L:6D for two days and then set into constant light. Cell samples will be taken every four hours starting at the time point that the cultures go into constant light. The DNA stain DAPI will be added to the cell suspension to track chromosome structure by visualization using fluorescence microscopy (Smith and Williams, 2006). To begin this work, I will need to learn the staining, microscopy, and data analysis techniques in the Williams laboratory. After developing these skills, I plan to bring the expertise back to the Biology Department at the Unviersity of St. Thomas as we have a fluroescence microscope such that raw chromosome compaction images will be taken at UST. UST does not have the capability at this time to analyze the raw images, therefore they will be sent to the Williams lab at the University of Utah for image deconvolution (where background fluorescence is removed) and compaction indeces will be calculated. Analysis comparison will be conducted between the different photoperiods with the 12L:12D photoperiod serving as the control. The data collected will be able to show whether the varying photoperiods cause the chromosome of the S. elongatus cells to compact at different time points from the standard 4 12L:12D photoperiod, and will demonstrate the mechanism of how behavior changes based upon photoperiod. If this occurs, it will support the data that my lab has already collected indicating that photoperiods do cause a phase shift in the cellular activity (see previous work). These results will help to determine if chromosome compaction can be changed by the amount and duration of light that the S. elongatus cells are receiving. Figure 3. Change in clock activity in response to photoperiod change in S. elongatus cells. Peak of activity occurs approximately 4 hours early in a 6L:18D (light blue) and approximately 4 hours late in a 18L:6D (dark blue) photoperiod relative to the 12L:12D control (green) (Dubis and Ditty, unpublished data). (c) Previous work: Preliminary work by undergraduates in my lab has begun to show the effects that various extreme photoperiods have on the circadian oscillator gene activity in cyanobacteria (Figure 3). Wild-type S. elongatus cultures were grown in 12L:12D, 6L:18D, and 18L:6D photoperiods for seven days. Results showed that increasing or decreasing the duration of light (18L:6D or 6L:18D) varied when the clock was most active (i.e., relative timing) by shifting the peak of activity by approximately 4 hours in delay and advance, respectively (Dubis and Ditty, 2007). In addition, Class 1 (day genes) and Class 2 (night genes) output genes that are controlled by the circadian clock have also been investigated. Interestingly, preliminary results have shown 5 that a Class 1 gene was affected by photoperiod, but the Class 2 gene was relatively insulated from photoperiod (Rhein et al., 2008). My students and I have studied the effects of photoperiod on the behavior of cyanobacteria. This previous work has now led our project into the realm of how the cells drive this change in behavior. Therefore, this proposed sabbatical project is a natural extension of my current research program. d) Specific professional goals: I have three specific goals for my sabbatical. First, I will learn the basic fluorescence microscopy and imaging techniques that have been used in the Williams lab for chromosome compaction studies. Second, I will apply this technique at UST to investigate the role of chromosome compaction in the cyanobacterial response to photoperiod. Third, I plan to disseminate these findings in the short-term at national meetings with the ultimate goal of publication of the scientific findings in peer-reviewed journals. All these goals will facilitate the expansion of my research program into new areas and techniques, which will allow me to provide novel research experiences for UST undergraduates. (e) Off-campus locations: I plan to spend approximately 2 weeks of the sabbatical in the laboratory of Dr. Stanly Williams at the University of Utah to learn the aforementioned microscopy techniques. Funding for the time in the Williams lab will be solicited through a UST Faculty Development Research Assistance Grant (RAG) or from grants from the Williams lab. The remainder of the sabbatical will be completed in my lab at UST. Raw images generated here at UST will be sent to the Williams lab for manipulation. A letter of support for this collaboration, along with the invitation to work in the Williams lab, is attached as Appendix 4. 6 APPENDIX 1. TIMETABLE FOR THE PROJECT 2011 January: Travel to the Williams laboratory at the University of Utah to learn the proper microscopy techniques to measure chromosome compaction. January through May: The end of January will be designated to microscope set up and troubleshooting here at UST. February through May will be used for the implementation of photoperiod experiments. This time period will also be used to send raw data images back to the Williams lab for data analysis. 7 APPENDIX 2. BIBLIOGRAPHY Works cited in narrative: Brasier MD, Green OR, Jephcoat AP, Kleppe AK, Van Kranendonk MJ, et al. 2002. Questioning the evidence for Earth’s oldest fossils. Nature 416:76-81. Cox G, Benson D, Dwarte DM. 1981. Ultra structure of a cave wall cyanophyte, Gloeocapsa NS 4. Archives of Microbiology 130:165-174. Ditty JL, Mackey SR, Johnson CH. Eds. 2009. Bacterial circadian programs. Berlin: SpringerVerlag. Ditty JL, Williams SB, Golden SS. 2003. A cyanobacterial circadian timing mechanism. Annual Reviews Genetics 37:513-543. Dubis JW, Ditty JL. 2007. Effects of photoperiod on the circadian mechanism in cyanobacteria. MN Academy of Sciences Annual Meeting. Hamline University, St. Paul, MN. Dunlap JC, Loros JJ, DeCoursey PJ. 2004. Chronobiology: Biological Timekeeping. Sunderland, MA: Sinauer. Johnson CH, Elliott JA, Foster R. 2003. Entrainment of Circadian Programs. Chronobiology International 20:741-774. Pittendrigh CS. 1981. Circadian systems: general perspective and entrainment. In Handbook of Behavioral Neurobiology: Biological Rhythms, ed. J Aschoff, pp. 57-80 and 95-124. New York: Plenum. Priscu JC, Fritsen CH, Adams EE, Giovannoni SJ, Paerl HW, et al. 1998. Perennial Antarctic lake ice: an oasis for life in a polar desert. Science 280:2095-2098. Rhein BA, Delaney KL, Dubis JW, Ditty JL. 2008. The effect of differing photoperiods on the output genes, psbA1 and purF of the cyanobacterium Synechococcus elongatus. North Central Branch American Society for Microbiology Meeting. St. Cloud State University, St. Cloud, MN. Smith RM, Williams SB. 2006. Circadian rhythms in gene transcription imparted by chromosome compaction in the cyanobacterium Synechococcus elongatus. Proceedings of the National Academy of Science USA 103:8564-8569. Pertinent references to the field of study Andersson CR, Tsinoremas NF, Shelton J, Lebedeva NV, Yarrow J, et al. 2000. Application of bioluminescence to the study of circadian rhythms in cyanobacteria. Methods in Enzymology 305: 527-542. Dunlap JC. 1999. Molecular bases for circadian clocks. Cell 96: 271-290. Golden SS, Ishiura M, Johnson CH, Kondo T. 1997. Cyanobacterial circadian rhythms. Annual Review of Plant Physiology Plant Molecular Biology 48:327–354. Ishiura M, Kutsuna S, Aoki S, Iwasaki H, Andersson CR, et al. 1998. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science 281: 15191523. Iwasaki H, Williams SB, Kitayama Y, Ishiura M, Golden SS, Kondo T. 2000. A KaiCinteracting sensory histidine kinase, SasA, necessary to sustain robust circadian oscillation in cyanobacteria. Cell 101: 223-233. Katayama M, Kondo T, Xiong J, Golden SS. 2003. ldpA encodes an iron-sulfur protein involved in light-dependent modulation of the circadian period in the cyanobacterium Synechococcus elongatus PCC 7942. Journal of Bacteriology 185: 1415-1422. Kondo T, Mori T, Lebedeva NV, Aoki S, Ishiura M, Golden SS. 1997. Circadian rhythms in rapidly dividing cyanobacteria. Science 275: 224-227. 8 Kondo T, Strayer CA, Kulkarni RD, Taylor W, Ishiura M, et al. 1993. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proceedings of the National Academy of Science USA 90: 5672-5676. Schmitz O, Katayama M, Williams SB, Kondo T, Golden SS. 2000. CikA, a bacteriophytochrome that resets the cyanobacterial circadian clock. Science 289:765-768. Vakonakis I, Klewer DA, Williams SB, Golden SS, LiWang AC. 2004. Structure of the Nterminal domain of the clock-associated histidine kinase SasA. Journal of Molecular Biology 342: 9–17. Whitton BA, Potts M. 2000. Introduction to Cyanobacteria. In The Ecology of Cyanobacteria, ed. BA Whitton, M Potts, pp. 1-11. Amsterdam: Klewer Academic Publishers. Xu Y, Mori T, Johnson CH. 2000. Circadian clock-protein expression in cyanobacteria: rhythms and phase setting. EMBO Journal 19: 3349-3357. 9