P181 Increasing use and efficacy of Rituximab for currently non
advertisement
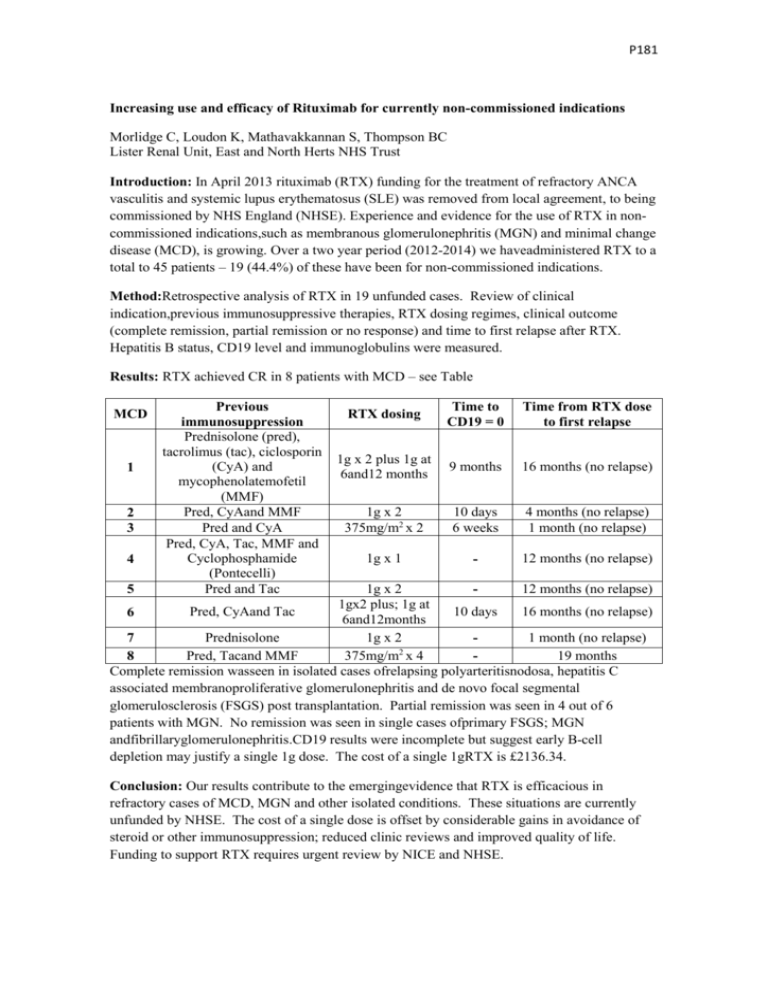
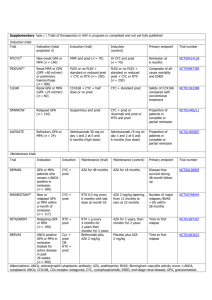
P181 Increasing use and efficacy of Rituximab for currently non-commissioned indications Morlidge C, Loudon K, Mathavakkannan S, Thompson BC Lister Renal Unit, East and North Herts NHS Trust Introduction: In April 2013 rituximab (RTX) funding for the treatment of refractory ANCA vasculitis and systemic lupus erythematosus (SLE) was removed from local agreement, to being commissioned by NHS England (NHSE). Experience and evidence for the use of RTX in noncommissioned indications,such as membranous glomerulonephritis (MGN) and minimal change disease (MCD), is growing. Over a two year period (2012-2014) we haveadministered RTX to a total to 45 patients – 19 (44.4%) of these have been for non-commissioned indications. Method:Retrospective analysis of RTX in 19 unfunded cases. Review of clinical indication,previous immunosuppressive therapies, RTX dosing regimes, clinical outcome (complete remission, partial remission or no response) and time to first relapse after RTX. Hepatitis B status, CD19 level and immunoglobulins were measured. Results: RTX achieved CR in 8 patients with MCD – see Table MCD 1 2 3 4 Previous immunosuppression Prednisolone (pred), tacrolimus (tac), ciclosporin (CyA) and mycophenolatemofetil (MMF) Pred, CyAand MMF Pred and CyA Pred, CyA, Tac, MMF and Cyclophosphamide (Pontecelli) Pred and Tac RTX dosing Time to CD19 = 0 Time from RTX dose to first relapse 1g x 2 plus 1g at 6and12 months 9 months 16 months (no relapse) 1g x 2 375mg/m2 x 2 10 days 6 weeks 4 months (no relapse) 1 month (no relapse) 1g x 1 - 12 months (no relapse) 1g x 2 12 months (no relapse) 1gx2 plus; 1g at Pred, CyAand Tac 10 days 16 months (no relapse) 6 6and12months Prednisolone 1g x 2 1 month (no relapse) 7 2 Pred, Tacand MMF 375mg/m x 4 19 months 8 Complete remission wasseen in isolated cases ofrelapsing polyarteritisnodosa, hepatitis C associated membranoproliferative glomerulonephritis and de novo focal segmental glomerulosclerosis (FSGS) post transplantation. Partial remission was seen in 4 out of 6 patients with MGN. No remission was seen in single cases ofprimary FSGS; MGN andfibrillaryglomerulonephritis.CD19 results were incomplete but suggest early B-cell depletion may justify a single 1g dose. The cost of a single 1gRTX is £2136.34. 5 Conclusion: Our results contribute to the emergingevidence that RTX is efficacious in refractory cases of MCD, MGN and other isolated conditions. These situations are currently unfunded by NHSE. The cost of a single dose is offset by considerable gains in avoidance of steroid or other immunosuppression; reduced clinic reviews and improved quality of life. Funding to support RTX requires urgent review by NICE and NHSE.