Article Macromolecules - iBlog Teacher Websites
advertisement

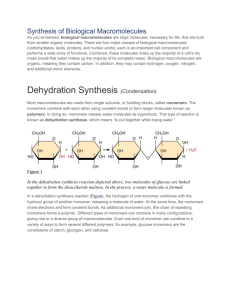
DO NOT WRITE DO NOT WRITE Macromolecules Article Read the article below and answer the questions that follow in your Interactive Notebook. Take your answers home, study them, I might have an open notes/ no notes quiz tomorrow depending on how you behave with the guest teacher. I will also check your answers when I walk in. Think back to what you ate for lunch. Did anything in there have a “Nutrition Facts” label on the back of it? If so, and if you had a look at that label, then you may already be familiar with several types of large biological molecules we’ll discuss here. If you’re wondering what something as weird-sounding as a “large biological molecule” is doing in your food, the answer is that it’s providing you with the building blocks you need to maintain your body – because your body is also made of large biological molecules! Just as you can be thought of as an assortment of atoms or a walking, talking bag of water, you can also be viewed as a collection of four major types of large biological molecules: carbohydrates (such as sugars), lipids (such as fats), proteins, and nucleic acids (such as DNA and RNA). That’s not to say that these are the only molecules in your body, but rather, that your most important large molecules can be divided into these groups. Together, the four groups of large biological molecules make up the majority of the dry weight of a cell. (Water, a small molecule, makes up the majority of the wet weight). Large biological molecules perform a wide range of jobs in an organism. Some carbohydrates store fuel for future energy needs, and some lipids are key structural components of cell membranes. Nucleic acids store and transfer hereditary information, much of which provides instructions for making proteins. Proteins themselves have perhaps the broadest range of functions: some provide structural support, but many are like little machines that carry out specific jobs in a cell, such as catalyzing metabolic reactions or receiving and transmitting signals. We’ll look in greater detail at carbohydrates, lipids, nucleic acids, and proteins a few articles down the road. Here, we’ll look a bit more at the key chemical reactions that build up and break down these molecules. Monomers and polymers Most large biological molecules are polymers, long chains made up of repeating molecular subunits, or building blocks, called monomers. If you think of a monomer as being like a bead, then you can think of a polymer as being like a bracelet or necklace, a series of beads strung together. Carbohydrates, nucleic acids, and proteins are all typically polymers. Because of their polymeric nature and their large (sometimes huge!) size, they are classified as macromolecules, large molecules made through the joining of smaller subunits. Lipids, which are not usually polymers and are smaller than the other three, are not formally considered to be macromolecules. However, many textbooks and sources use the term “macromolecule” more loosely, as a general name for the four types of large biological molecules. This is just a naming difference, so don’t get too hung up on it. Just remember that lipids are one of the four main types of large biological molecules, but that they don’t generally form polymers. DO NOT WRITE DO NOT WRITE Dehydration synthesis How do you go from monomers to polymers? Large biological molecules typically assemble via dehydration synthesis reactions, in which one monomer forms a covalent bond to another monomer (or growing chain of monomers), releasing a water molecule in the process. You can remember what happens by the name of the reaction: dehydration, for the loss of the water molecule, and synthesis, for the formation of a new bond. Dehydration synthesis reaction between two molecules of glucose, forming a molecule of maltose with the release of a water molecule. In the dehydration synthesis reaction above, two molecules of the sugar glucose combine to form a single molecule of the sugar maltose. One of the glucoses loses an H, the other loses an OH group, and a water molecule is released as a new covalent bond forms between the glucose molecules. As additional monomers join by the same process, the chain can get longer and longer and form a polymer. When you first think about polymers, it might seem like there’s not much room for variation. Just a bunch of identical monomers, stuck together to form a long and boring chain. But nothing could be further from the reality! Biological polymers are actually almost dazzlingly diverse. For one thing, polymers are made out of monomers, but not all monomers of a given type are identical. Carbohydrates, nucleic acids, and proteins all have multiple different types of monomers, which you can think of as letters of an alphabet. These letters can be arranged in different ways to spell different words, write different sentences, and even produce completely distinct paragraphs. For instance, there are 20 common amino acid “letters” in the alphabet used to spell out proteins. In addition, even a single kind of monomer may be able to combine in a variety of ways to form different polymers. For example, starch, glycogen, and cellulose are all made up of glucose monomers but have different bonding and branching patterns. Hydrolysis In biological systems, what gets built up must eventually break down. So, how do polymers turn back into monomers (for instance, when the body needs to recycle one molecule to build a different one)? Polymers are broken down into monomers via hydrolysis reactions, in which a bond is broken by addition of a water molecule. During a hydrolysis reaction, a molecule composed of multiple subunits is DO NOT WRITE DO NOT WRITE split in two: one of the new molecules gains a hydrogen atom, while the other gains a hydroxyl (-OH) group, both of which are donated by water. This is simply the reverse of a dehydration synthesis reaction, and it releases a monomer that can be used in building a new polymer. For example, the hydrolysis reaction below splits up maltose to release two glucose monomers, and is the reverse of the dehydration synthesis reaction shown above. Hydrolysis of maltose, in which a molecule of maltose combines with a molecule of water, resulting in the formation of two glucose monomers. Dehydration synthesis reactions build molecules up and generally require energy, while hydrolysis reactions break molecules down and generally release energy. Carbohydrates, proteins, and nucleic acids are built up and broken down via these types of reactions, although the monomers involved are different in each case. (In a cell, nucleic acids actually aren't polymerized via dehydration synthesis; we’ll examine how they're assembled in the article on nucleic acids.) Dehydration synthesis reactions are also involved in the assembly of certain types of lipids, even though the lipids are not polymers. In the body, enzymes catalyze, or speed up, both the dehydration synthesis and hydrolysis reactions. Enzymes involved in breaking bonds are often given names that end with -ase: for instance, the maltase enzyme breaks down maltose, lipases break down lipids, and peptidases break down proteins (also known as polypeptides, as we’ll see in the article on proteins). As food travels through your digestive system – in fact, from the moment it hits your saliva – it is being worked over by enzymes like these, which break down large biological molecules to release the smaller building blocks that can be readily absorbed and used by the body. DO NOT WRITE DO NOT WRITE Questions: (Answers go in your INB, you NEED to Write your questions down) 1- How is the "nutrition facts" label related to my building blocks of life? 2- What makes up the majority of the dry weight of a cell? 3- What is the function of carbohydrates? 4- What is the function of proteins? 5- What is the function of lipids? 6- What is the function of Nucleic Acids? 7- What is the difference between a monomer and a polymer? 8- How do monomers join to make polymers? Name and explain the process 9- Draw out the dehydration synthesis equation of Maltose 10- Besides Maltose, what is the other product of dehydration synthesis? 11- Can you make a conclusion about why we call this reaction "dehydration synthesis"? 12- What makes starch, glycogen, and cellulose different? 13- How do polymers break to make monomers? Name and explain the process 14- Draw out the Hydrolysis equation of Maltose 15- What is added to Maltose to produce the two glucoses? 16- How do the two reactions above utilize energy differently? 17- What is the function of enzymes? Give an example. 18- Which statement about the synthesis of lipids best supports the fact that lipids are not polymers? Copy the sentence down 19- What is a new idea that you learned from this article. 20- Did you copy all the questions in your notebook? Label the page as: Macromolecules Article: Questions and Answers