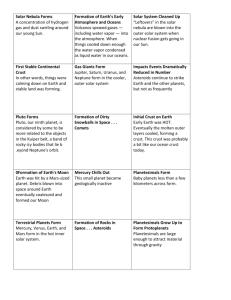
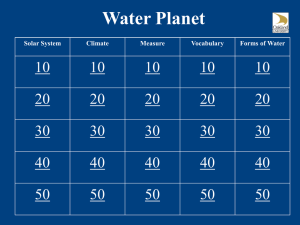
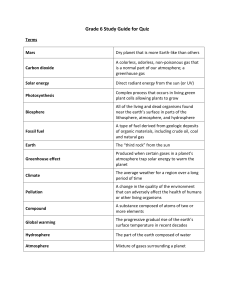
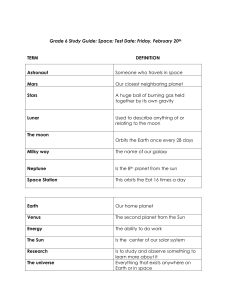
File - Philosophy, Theology, History, Science, Big
advertisement

Increases in Oxygen Prepare Earth for Complex Life June 30th, 2010 By Dr. Jeff Zweerink One of Earth’s most remarkable attributes is the permanent oxygen component of the planet’s atmosphere. Oxygen’s reactivity makes it an efficient energy source for life, but it also means oxygen would disappear quickly without a continuous resupply. Atmospheric oxygen increased dramatically during two different periods in Earth’s history. Yet these increases occurred only because of a complex and elegant interplay of geological, astronomical, biological, atmospheric, and chemical processes. For most of Earth’s history its atmosphere contained no oxygen. Then, just over two billion years ago, oxygen gained a permanent foothold, though at a fraction of today’s concentrations. Even greater jumps in oxygen content occurred between 600 and 800 million years ago. These jumps resulted in long-standing consequences. First, they were often accompanied by intense ice ages where glaciers advanced close to the equator. Scientists believe these aptly-named “snowball Earth” events may have occurred because increased oxygen levels converted methane, a strong atmospheric greenhouse gas, into the less potent carbon dioxide. As catastrophic as these snowball events were, the changes in Earth’s atmosphere were necessary to compensate for the Sun’s steadily increasing luminosity. Fortunately, aspects of biological activity prevented the glaciations from destroying Earth’s capacity to support life. Most importantly, the oxygen jumps ushered in a dramatic rise in life’s complexity. Previous research indicated that changes in the plate tectonic activity—specifically the first formation of tall mountains—increased the supply of molybdenum to the oceans and that, in turn, boosted biological activity. Now it appears that the changes in biological activity (both the increase in number and complexity) also required a boosted phosphorous supply. According to research recently published in Astrobiology, phosphorite deposits across the globe correspond to Earth’s two great oxygenation events. 1 The model outlined in the paper argues that tectonic processes produced a greater (and higher) continental landmass. The weathering of this landmass transported phosphorous to the oceans, causing a dramatic rise in primary biological production. After the last increase in oxygen around 650 million years ago Earth could finally support complex, multicellular organisms. We at RTB argue that any mechanism exhibiting complex, integrated actions that bring about a specified outcome is designed. Studies of Earth’s history reveal highly orchestrated interplay between astronomical, geological, biological, atmospheric, and chemical processes that transform the planet from an uninhabitable wasteland to a place teeming with advanced life. The implications of design are overwhelming. Endnotes: 1. Dominic Papineau, “Global Biogeochemical Changes at Both Ends of the Proterozoic: Insights from Phosphorites,” Astrobiology 10 (April 19, 2010): 165–81. Too Much Oxygen in the Past September 22nd, 2010 By Dr. Jeff Zweerink Oxygen Spikes Jumpstart Life's Complexity and Size March 1st, 2010 By Dr. Jeff Zweerink Since life first appeared on Earth, the size of the largest organisms increased in size by a factor of 10 quadrillion (1016 or 10,000,000,000,000,000). Two sudden bursts, each showing an organism volume increase by a factor of one million (106), account for most this growth. 1 Both bursts occurred after a significant change in the quantity of oxygen in Earth’s atmosphere. The latter oxygenation event occurred just prior to the Cambrian explosion. Research into the timing and stability of the first oxygenation event provides more evidence supporting RTB’s creation model, which predicts that complex life would appear suddenly and early in Earth’s history. Timing of First Oxygenation Event The geological column records the history of how life changed as time progressed. A number of geological signatures indicate oxygen appeared as a permanent component in Earth’s atmosphere 2.4 billion years ago. However, evidence shows that photosynthetic organisms arrived on the scene at least 100 million years earlier. Studies of nitrogen cycling may explain the delay.2 The presence of oxygen affects how nitrogen interacts with its environment. Lacking oxygen, a specific ratio of nitrogen isotopes is deposited on the ocean floor. Adding oxygen to the mix will increase the amount of heavier nitrogen (15N) compared to lighter nitrogen (14N and 13N). Around 2.7 billion years ago, the amount of heavier nitrogen increased by a detectable amount, providing evidence that photosynthetic organisms were producing oxygen. A second increase 150 million years later indicates that the nitrogen cycle changed again, thus demonstrating some instability. During this latter increase the amount of “fixed” nitrogen decreased. Many organisms cannot use atmospheric nitrogen (N2) for energy production but rely on “fixed” nitrogen, primarily in the form of ammonia. The lack of fixed nitrogen then limits the productivity of the photosynthetic organisms, keeping the amount of oxygen in the atmosphere low. This research highlights how difficult it is to effect major changes in a planet’s atmospheric chemistry. Even with the advent of oxygen producing organisms, a permanent component of atmospheric oxygen was delayed hundreds of millions of years––suggesting that the agent of change was beyond natural. Stability of First Oxygenation Event Most geological signatures point to the arrival of eukaryotes (animals, plants, fungi) on Earth around 1.6–2.1 billion years ago. In contrast to the prokaryotes (bacteria, archaea) dating back to 3.8 billion years, eukaryotes exhibit far more internal structure and can grow much larger. It was the advent of eukaryotes that accounts for the first rapid increase of organism size. Even though a growing body of evidence shows that Earth’s atmosphere contained oxygen starting 2.4 billion years ago, more detailed studies hint at instabilities during its initial stages. 3 This instability may explain why the first dramatic increase in organism size and complexity did not occur for roughly half-a-billion years after the first appearance of oxygen. Evidence for this atmospheric oxygen instability comes from the same element that makes (old) bumpers shine and steel stainless, namely chromium. In an atmosphere without oxygen, chromium remains locked in the continental crust. In the presence of oxygen, chemical reactions extract chromium and lead to weathering processes that then transport it to the ocean. Additionally, these processes alter chromium’s isotopic composition in a way that can be used to trace the presence of oxygen in the atmosphere. A team of scientists analyzed these chromium isotopes in ancient sea sediments. Their research revealed chromium isotope fractionation in formations deposited before the great oxygenation event around 2.4 billion years ago. This find indicates that the oxygen levels rose for a geologically brief period of time (a couple million years). However, in more recent formations, around 1.9 billion years ago, no fractionation occurs, pointing to a lack of oxygen in Earth’s atmosphere. Over the next hundred million years atmospheric oxygen permanently increased, preceding the appearance of eukaryotic life and the associated factor of a million jump in body size. If naturalistic processes triggered the formation of the more complex eukaryotic life, scientists would expect to see some fossil traces of eukaryotes during the earlier increases in oxygen. Instead, eukaryotes don’t appear until oxygen gains a permanent foothold in the atmosphere. This exquisite timing is consistent with the notion of a carefully designed plan to bring about a planet maximized for human habitability. __________ Jonathan L. Payne et al., “Two-phase Increase in the Maximum Size of Life over 3.5 Billion Years Reflects Biological Innovation and Environmental Opportunity,” Proceedings of the National Academy of Sciences, USA 106 (January 6, 2009): 24–27. http://www.pnas.org/content/106/1/24.full?sid=92f9f43c-f811-465e-a07c903052793240 1 2 Linda V. Godfrey and Paul G. Falkowski, “The Cycling and Redox State of Nitrogen in the Archaean Ocean,” Nature Geoscience 2 (October 1, 2009): 725–29. http://www.nature.com/ngeo/journal/v2/n10/full/ngeo633.html 3 Robert Frei et al., “Fluctuations in Precambrian Atmospheric Oxygenation Recorded by Chromium Isotopes,” Nature 461 (September 10, 2009): 250–53. http://www.pnas.org/content/106/1/24.full?sid=92f9f43c-f811465e-a07c-903052793240 Subjects: Geophysical Design “This fire needs more wood!” said my oldest daughter as we sat around the campfire after setting up in the rain. I couldn’t agree more. One of my favorite activities is roasting s’mores over the campfire in the cool of the evening. Everyone knows you need graham crackers, chocolate squares, and marshmallows to make s’mores. But one essential “ingredient” that often goes unnoticed is the atmosphere’s oxygen content. For the last 50 million years, this important gas comprised 20 percent of the atmosphere. New research indicates that this value fluctuated dramatically in earlier times. Attempts to measure Earth’s past oxygen content often give conflicting results. This difficulty arises because scientists cannot directly measure the ancient atmospheric gases but must use proxies instead. Numerous variables affect the geological record, oxygen being just one of those variables. However, a team of scientists recently found a way to control for all the other variables by using coal as a proxy for past oxygen content. Without oxygen, nothing burns—but with enough oxygen, even wet objects readily combust. Thus, the researchers were able to use charcoal (burned organic matter) formed in water-rich environments as the proxy. The amount of charcoal in coal depends primarily on the amount of gaseous oxygen available and coal’s economic value means a large database of charcoal compositions already exists. The information in this database demonstrates that even with dramatic climate changes over the last few million years the amount of coal remained relatively uniform. This matches the expectation that the oxygen content of the atmosphere remained constant over the past 50 million years. However, over the last 400 million years, the oxygen showed dramatic increases and decreases compared to current values.1 Past life on Earth may have been well adapted to these changes, but similar changes today would cause significant problems for humanity. Too much oxygen in the atmosphere leads to explosive and destructive wildfires. Too little oxygen means less energy is available to fuel biochemical reactions inside largebodied organisms, like humans. An increasing body of evidence shows that Earth’s environment changed numerous times in ways that altered the kinds of life able to survive on the planet. Yet humanity arrived on the scene during a stable period when the atmospheric oxygen met all the criteria that advanced life requires. Such fine-timing follows if a supernatural Designer is preparing a place for human life. Subjects: Geophysical Design More Evidence for the Design of Earthquake Activity August 18th, 2008 By Dr. Hugh Ross Faulting, generated by active and widespread tectonics, allowed a youthful Earth to support diverse and abundant life. In the December 2007 issue of Astrobiology Stanford University geophysicists Norman H. Sleep and Mark D. Zoback note that the higher tectonic activity during Earth’s early history could have played a key role in cycling critically important nutrients and energy sources for life.1 The production of numerous small faults in the brittle primordial crust released trapped nutrients. Such faults could also release pockets of methane gas and molecular hydrogen. The methane and hydrogen could then provide crucial energy sources for nonphotosynthetic life. Finally, the production of faults could bring water to otherwise arid habitats, such as rocks far below Earth’s surface. Faulting, generated by active and widespread tectonics, allowed a youthful Earth to support diverse and abundant life. This enhanced diversity and abundance of life quickly transformed Earth’s surface into an environment safe for advanced life. Also, the buildup of biodeposits for the support of human civilization occurred more rapidly due to active tectonics. The more rapid preparation of Earth for humanity is critical. Without such rapid preparation, humans could not come upon the terrestrial scene before the Sun’s increasing luminosity would make their presence impossible (due to excessive heat).2 Thus, yet one more reason exists to thank God for His supernatural design of Earth’s tectonics. Subjects: Biodeposits, Extrasolar Planets, First Life on Earth, Geophysical Design, Habitable Planets, Natural Disasters, Plate Tectonics, Solar System Design, TCM - Cosmic Design Subduction Design November 3rd, 2008 By Phil Chien 11/3/2008 by Dr. Hugh Ross Stanford University geophysicist Norman Sleep has outlined some new constraints on habitable planets.1 He explains how the possible existence of advanced life crucially depends upon a planet maintaining efficient plate tectonics for billions of years. Without such plate tectonics several nutrient-recycling processes, critical for advanced life, cannot be sustained. Efficient plate tectonics are also essential for transforming a planet’s surface into a mix of oceans and continents. Both the nutrient recycling and the development of continental landmasses require a high rate of subduction. Subduction is the sliding of one tectonic plate under another. For subduction to take place, the tectonic plates need to slip in friction at the fault zones. Also, the lithosphere within the crustal slab that is slipping under another crustal slab needs to bend with a specified strain. An overarching design requirement for advanced life, then, is that the rate of subduction must be fine-tuned. Too low of a subduction rate would lead to inadequate nutrient recycling and inadequate buildup of continents. (If the buildup rate is much less than the erosion rate, the continents will disappear.) Too high of a subduction rate would disturb the ecosystems of advanced life and challenge the development of global high-technology civilization. To sustain the subduction rate at the just-right level that advanced life needs means that the sliding friction between crustal plates at the subduction zones must be maintained at just-right levels. Also, the crustal slabs undergoing subduction need to bend at the just-right levels and rates. All this fine-tuning adds to the growing weight of evidence that a supernatural, super-intelligent Creator is necessary to explain all the characteristics of Earth that must be present in order for the planet to be habitable by advanced life. It also implies that, unless the Creator has intervened in other places in the cosmos, astronomers will not find advanced-life habitable planets elsewhere in the Milky Way Galaxy or in any other galaxy. 1. Norman Sleep, “Tectonics and Habitability of Super-Earths,” Astrobiology 8 (April 2008): 395. Subjects: Geology and the Bible, Geophysical Design, Plate Tectonics Earth Just Barely Large Enough February 13th, 2008 By Dr. Jeff Zweerink All the recent talk about global warming highlights one critical characteristic of Earth that makes the planet habitable, namely plate tectonics. As I discussed two weeks ago, Venus and Earth are remarkably similar in terms of their size and composition. Both probably started out with large oceans of water. While Earth continues to maintain a global temperature that supports a vital stable water cycle, the surface of Venus is bone dry with temperatures near 800oF. Unlike on Venus, Earth’s plate tectonics still operate and therefore perform the important function of removing greenhouse gases from Earth’s atmosphere. Without plate tectonics, a dense life-suffocating carbon dioxide atmosphere would surround Earth. Research presented at the 211th meeting of the American Astronomical Society (AAS) puts tight constraints on how large a planet must be to sustain long-standing plate tectonics. Essentially, all rocky planets larger than twice the size of Earth will experience plate tectonics. However, as a consequence of thinner tectonic plates and greater geological stresses these “super-Earths” would experience more vigorous plate tectonics. Furthermore, the research illuminates two limits for habitable, tectonically active planets. First, any planet larger than ten times Earth’s mass will attract a dense hydrogen and helium atmosphere, like the gas giants in our solar system. Consequently these planets cannot be habitable. On the other end of the spectrum, Earth is barely large enough to sustain plate tectonics. Earth’s large, liquid water oceans and abundant interior water both lubricate tectonic movements and give Earth’s interior the necessary characteristics to support tectonic activity. Thus, for any planet closer to Earth’s mass, the planet must exhibit the facilitating properties of water. This research implies that scientists will discover more potentially habitable planets. However, the tectonic activity on these super-Earths will be far more destructive than on Earth. Thus, RTB’s creation model predicts that planets much larger than Earth will prove uninhabitable—or at least incapable of supporting human life. Subjects: Big Bang, Earth/Moon Design, Multiverse EXTRA! EXTRA! READ ALL ABOUT IT! July 1st, 2007 By Dr. Jeff Zweerink You’re Standing on a Floating Plate This just in—ice floats in water! Unlike most materials, as liquid water cools to near its freezing point, its density decreases and then expands as it freezes. Thus, colder water and any ice float on the warmer liquid water below. Seems anticlimactic, doesn’t it? However, if water did not possess this unusual property, Earth’s habitability would dramatically decrease. Ponds, lakes, streams, rivers, and possibly even oceans would freeze completely solid—not just on the surface—more regularly and take far longer to thaw after temperatures rise. Amazingly, a similar phenomenon occurring deeper in the Earth may be responsible for enabling our planet to maintain the long-standing plate tectonics so critical for enduring life. Recall that Earth consists of a shallow crust, mantle, and an outer and inner core. Moving from the crust toward the core, most of the relevant materials in Earth’s interior absorb water more readily (because both temperature and pressure increase with depth). One material, a mineral called aluminous orthopyroxene found throughout the Earth, exhibits peculiar behavior in the upper mantle (just below the crust) called the asthenosphere. In this region, aluminous orthopyroxene’s capacity to dissolve water drops dramatically, but increases again at greater depths. Therefore, the materials in this region of the mantle absorb less water than those above and below with the consequence that a large abundance of “hydrous” melt exists in the asthenosphere. Think of a cracker sandwich with jelly (as the asthenosphere) in the middle. Why does all this matter? The melt provides two important functions. First, it significantly weakens the asthenosphere such that it becomes more malleable and fluid. Second, the melt absorbs a tremendous amount of water compared to the other mantle components and, consequently, it dehydrates the region above the asthenosphere called the lithosphere (or crust). The lithosphere is comprised of the crustal plates that migrate over the surface of the Earth. Taken together, these two effects lead to a process—otherwise known as plate tectonics—where the rigid crustal plates “float” on a weakened and malleable asthenosphere. Another mineral called olivine constitutes the dominant component of Earth’s mantle, and the solubility of water in olivine and orthopyroxene is similar—at least in the absence of aluminum. Until recently, scientists believed that olivine controlled the water storage capacity of Earth’s interior. However, the addition of aluminum increases the solubility of orthopyroxene nearly one hundred times. Consequently, scientists now recognize aluminous orthopyroxene, with its unusual solubility characteristics, as the controlling material responsible for Earth’s ideal tectonic activity. This discovery also implies constraints on planet sizes where plate tectonics can occur. On planets that are too large (or small), the location of the asthenosphere will be too deep (or shallow) to permit the necessary crustal plate movement. As the authors of the article conclude, the existence of plate tectonics “is possible only in a planet with a water-bearing mantle” (that also contains sufficient aluminum). Such results echo the words of the Creator who fashioned Earth not as “a waste place, but formed it to be inhabited.” Subjects: Geophysical Design Plains, Minerals, and Mountain Building August 1st, 2011 By Dr. Jeff Zweerink Living in Missouri, family vacations often took us out west to Colorado and other Rocky Mountain states. After a long, flat drive across Kansas and almost half of Colorado, the Rockies–– almost without warning––pop out of the ground. I still remember the stark contrast of the majestic peaks against the spacious plains. It’s as if some rocks are meant to form mountains and others remain flat. But why? Recent research begins to answer that question and also reveals how finely tuned tectonic activity plays a critical role in Earth’s habitability. Is it all by lucky accident or divine design? Eight major plates (and dozens of smaller plates) form Earth’s crust. These plates slowly move around like giant ice floes on a cold sea. If “crust is crust,” one might expect a different transition between the Rocky Mountains and the Great Plains. As the North American plate drifts away from the African and Eurasian plates, it continually collides with more western plates. The Farallon plate has almost slid completely underneath the North American plate and the Pacific plate is beginning to do so. This continual collision dissipates an enormous amount of energy that causes the plates to deform. Specifically, the North American plate buckled over vast distances, thus forming the Rockies as well as the Sierra Nevada, Cascades, and other West Coast mountain ranges. Collectively, the mountains running up and down the West Coast of the Americas is referred to as the American Cordillera. One outstanding question is why the mountain formation extended hundreds of miles toward the interior of the continent before abruptly stopping. Quartz Builds Mountains… Part of the answer rests in the types of minerals that comprise different sections of the North American crust, but isolating the specific differences is not trivial. Many factors contribute to how much stress geological formations experience and how they respond to that stress. Some of the main contributors include rock type, temperature, and the presence of unbound water. A pair of geologists found a way to sort out the differences and isolate the influence of rock type.1 One measurable quantity, the ratio of compression and shear velocities (the rates at which seismic waves move through the Earth), correlates strongly with rock type—specifically, quartz abundance—but weakly with temperature. Thus, measuring this ratio will determine quartz content of a region of crust regardless of its temperature. Utilizing seismic receiver, gravity, and surface heat flow measurements from EarthScope for the Cordillera, the researchers found a strong correlation of high quartz content with increased temperatures and deformation. The most straightforward interpretation of this correlation means that the structurally weak (compared to other crustal minerals) quartz gives way under stress and generates heat localized to the quartz-rich crust. The heating results in more unbound water and further weakening of the crust. This feedback cycle results in large deformations (mountains) and heating in quartz-rich regions. Not only does this research help explain why mountains seem to rise abruptly out of the plains, it also solves another long-standing observation. While mountains grow and erode over geological timescales, they seem to repeatedly form in the same sections of crust. If quartz-rich crust deforms more readily than other compositions, one would expect this observed behavior. …But Water Moves Continents In order for mountains to grow, the crustal plates must be free to move around the Earth’s surface and collide with one another. This requires a mechanism that (1) makes the plates rigid (like the frozen water of an ice floe); and (2) softens the upper mantle so that the plates slide easily (like the water underneath). Furthermore, all this must happen at the proper depth or else the plates will be too thick or too thin for the tectonic activity to provide its life-essential functions. Research continues to demonstrate how critically water facilitates these characteristics in Earth’s crust. Just as quartz allows the crust to deform and build mountains, water’s interaction with the rocks and minerals makes the crustal plates more rigid and softens the top of the underlying mantle—known as the asthenosphere. And on Earth, it does this at the just-right depth for the proper amount of tectonic activity. Pressure and temperature increase when moving from the surface toward the center of Earth. Normally, these increases result in a greater capacity of the associated rocks and minerals to dissolve water. However, a team of geophysicists noted how a major change occurs at the temperatures and pressures associated with depths 55 miles below the ocean floor or at the asthenosphere-crust (AC) boundary.2 Rather than the crustal rocks dissolving more water as the temperature and pressure increase, the water facilitates partial melting of the rock. Above this depth, the water-containing minerals exhibit a stable phase that makes them solid and rigid. Below this depth, the partial melting of the rocks means the asthenosphere takes on a more fluid nature and allows convection to transport minerals from the lower asthenosphere toward the bottom of the crust. This transport helps explain the composition of rocks measured in the mid-ocean ridges where the ocean plates separate and new material from the asthenosphere fills the gap. The water content of this new rock matches the value expected from the lab measurements simulating conditions of the AC boundary. These results further affirm how important it is for a planet to be of the right size and to have the right water content in order for plate tectonics to operate properly. Implications of all this Movement Without plate tectonics, Earth’s land would erode away and all the life-essential nutrients would reside far below the ocean’s surface. Eventually, Earth would lose all its water. Similarly, if Earth lost all its water, plate tectonics would grind to a halt. This new research demonstrates that without sufficient quartz in Earth’s crust, mountain formation might not happen or it might not build the tall mountains that helped prepare for advanced life. And a growing body of evidence indicates that a planet must be of the just-right size so that the boundary between the asthenosphere and crust occurs at the right depth for plate tectonics to operate at the just-right efficiency. Significantly more tectonic activity would make advanced civilization far more difficult, if not impossible. Less activity would not allow the continents and mountains to form quickly enough or to recycle the nutrients life requires. Yet, we live on a planet that meets all these conditions in such a way that advanced human civilization thrives and does so with an abundance and diversity of life almost beyond comprehension. It seems like Someone designed Earth for just this purpose. That’s something to ponder and be thankful for when a mountain range, in all its splendor, is a part of your next trip. 1. Anthony R. Lowry and Marta Pérez-Gussinyé, “The Role of Crustal Quartz in Controlling Cordilleran Deformation,” Nature 471 (March 17, 2011): 353–57. 2. David H. Green et al., “Water and Its Influence on the Lithosphere–Asthenosphere Boundary,” Nature 467 (September 23, 2010): 448–51. Subjects: Geophysical Design The Concentration of Metals for Humanity's Benefit May 11th, 2009 By Dr. Hugh Ross Without concentrated ores of insoluble metals embedded into Earth's crust, human civilization would've never advanced beyond a stone-age culture. Today we have the ability to glean unconcentrated metals from rocks, soils, and oceans. The technology to do so, however, wouldn't exist without humanity's prior access to concentrated ores. Most of the concentration of Earth's ores resulted from bacterial activity. For example, over the course of hundreds of millions (in some cases billions) of years, different species of sulfate-reducing bacteria fed on dilute soluble metal compounds, converting these compounds into insoluble forms. The decayed residues of the bacteria yielded the concentrated ores. An international team of geologists recently elucidated another metal-concentrating mechanism unique to Earth, at least in its extent.1 This mechanism is the concentration of metals in hydrothermal solutions. Hydrothermal fluid flow moving through a mineralized part of Earth's crust will transport dissolved metals. If a mechanism exists to precipitate out these metals in insoluble form within a confined local region for a long enough period of time, an economically mineable ore deposit will form. That is, hydrothermal fluid flow under just-right conditions can scavenge metals at a low concentration level from a large volume of rock and then concentrate those metals into a much smaller rock volume. In their research paper, the team of geologists proposed2 a number of mechanisms known to operate within Earth's crust that could explain the precipitation. They then performed a set of experiments in which they used "laser ablation inductively coupled plasma mass spectrometry" to determine the enrichment factors for these mechanisms. They demonstrated that hydrothermal fluid flow could enrich the concentration of metals like zinc, lead, and copper by at least a factor of a thousand. They also showed that ore deposits formed by hydrothermal fluid flows at or above these concentration levels exist throughout Earth's crust. The necessary just-right precipitation conditions needed to yield such high concentrations demand extraordinary fine-tuning. That such ore deposits are common in Earth's crust strongly suggests supernatural design. Evidently, the Creator used a variety of carefully designed mechanisms, both organic and inorganic, to concentrate and preserve for humanity's benefit a bountiful treasure of concentrated insoluble ore deposits. 1. 2. Jamie J. Wilkinson et al., "Anomalously Metal-Rich Fluids Form Hydrothermal Ore Deposits," Science 323 (February 6, 2009): 764-67. Robert J. Bodnar, "Heavy Metals or Punk Rocks?" Science 323 (February 6, 2009): 724-25. Subjects: Geophysical Design Too Much Sulfur October 6th, 2008 By Dr. Hugh Ross Recent studies conducted on Venus and Mars illustrate just how carefully fine-tuned a planet’s abundance of sulfur must be for life to be possible. Sulfur plays a crucial role in life chemistry. This fact became personal for me a year ago when I was diagnosed as sulfur deficient. Many protein functions crucially depend on sulfur. Fortunately, most agricultural soils contain plenty of sulfur that vegetables, like onions and garlic, readily absorb. So, now that my sulfur deficiency has been resolved, my family has requested that I back off on the garlic. Too much sulfur, however, can lead to consequences far more devastating than bad breath. Acid rain results from industrial activity pumping too much sulfur compounds into the atmosphere. Many life-essential metabolic reactions are adversely affected by the acidic conditions brought about by sulfur pollution. One reason life thrives on Earth is because of its low sulfur-water ratio. For Earth to have both such a low ratio and a relatively thin atmosphere is nothing short of miraculous. Earth’s sister rocky planets, Venus and Mars, help highlight Earth’s amazingly benign conditions for life. Venus, like Earth, is sulfur poor, but it has no water and, despite being less massive than Earth, its atmosphere is ninety times more massive than Earth’s. Mars has a thin atmosphere but the Mars Exploration Rover Missions, Spirit and Opportunity, have confirmed and greatly extended the evidence for the dominant role of sulfur in Mars’ geochemical processes.1 Astrobiologists now acknowledge that the high sulfur-water ratio on Mars is toxic, which rules out any naturalistic origin-of-life scenario. Astrobiologists now also understand how Mars attained its high sulfur-water ratio. For any rocky planet, its crustal sulfur-water ratio is dictated by three factors: planetary accretion resources, the degree of core formation, and igneous evolution. Earth accreted less sulfur than Mars and most of the sulfur it did accrete— because of some extraordinary mass collision events—got incorporated into the planet’s interior.2 Those same extraordinary mass collision events also explain how Earth, as massive as it is, ended up with such a thin atmosphere.3 The lander missions on Mars and Venus illuminate a Christian apologetics principle. They demonstrate that the more we learn about the physics and chemistry of other planets, the more evidence we accumulate for the supernatural, super-intelligent design of Earth for the benefit of all life, both simple and complex. Subjects: Extrasolar Planets, Galaxy Design, Geophysical Design, Habitable Planets, Life Design, Life on Other Planets, Mars, Prebiotic Chemistry, SETI, Solar System Design, TCM - Cosmic Design, TCM - Life Design, TCM Life's Origin The Creation of Minerals April 20th, 2009 By Dr. Hugh Ross The debate over creation and evolution has taken a significant evolutionary leap. In this year that marks Charles Darwin's 200th birthday and the 150th anniversary of the publication of his book, On the Origin of Species,1 the debate over creation and evolution has taken a significant evolutionary leap. An American-Canadian team of geologists and geophysicists recently published a paper in which they argue that minerals on and in the crust of Earth have evolved.2 They make the claim that, in a manner similar to the history of life, new minerals adapt or evolve with changing environmental conditions throughout geologic time.3 In their attempts to provide an evolutionary explanation for the mineral history of Earth's crust the research team inadvertently delivers much new evidence for creation. They point out that the dust particles in the prestellar molecular cloud from which the solar system formed contained only about a dozen different minerals. Gravitational clumping within the molecular cloud led to the formation of a proto-Sun surrounded by a protoplanetary disk. Then, careful fine-tuning of the protoplanetary disk's characteristics resulted in the meteorites ending up with 60 different mineral species, out of which Earth formed. Subsequent aqueous and thermal alteration of the meteorites and asteroidal accretion and differentiation, under just-right conditions, caused the 60 minerals to increase to 250. Once Earth had fully formed with a stable crust and ocean in place, biological processes produced explosive advances in Earth's surface mineralogy. In fact, the research team demonstrated that with each big bang of life (sudden, widespread radiation of new life-forms) a mineralogy big bang followed. They show that the most dramatic biomineral explosion by far took place following the Cambrian explosion event some 543 million years ago. According to the research team life plays a crucial and unique role in Earth's surface mineralogy. Unlike any other chemical pathway, living systems can generate far-from-equilibrium conditions. Thus, the enormous diversity and abundance of life throughout the past 543 million years resulted in an especially great variety of far-from-equilibrium chemical circumstances. Thanks to the way life was introduced on Earth, the early 250 mineral species have exploded to the present 4,300 known mineral species. And because of this abundance, humans possessed all the necessary mineral resources to easily launch and sustain global, high-technology civilization. RTB's creation model asserts that without the supernatural planning, design, and control with which the Creator formed the planet and introduced and sustained life, humanity would have been bereft of the necessary mineral reserves, in addition to biological and biodeposit resources. Just as the long progression of life on Earth from simple-to-complex-and-diverse testifies of the handiwork of a super-intelligent Creator rather than just mere natural processes, likewise the growth in the number and complexity of mineral species on Earth's surface over the past four billion years gives evidence for the work of the same Creator. 4 Subjects: Biodeposits, Geophysical Design, Life Design What If There Were No Hurricanes? January 1st, 2006 By Administrator 1/1/2006 by Dr. David Rogstad Those who have suffered through the recent North Atlantic hurricane season would probably prefer nothing more than an afternoon shower ever again. High death tolls, staggering property losses, and frightening devastation earn these tropical cyclones their reputation as "acts of God." People everywhere wonder, "If God is so great and has designed the world, why would hurricanes be a part of His good creation?" This question deserves a compassionate, thorough answer,1 but this short article briefly addresses one aspect of such a complicated issue. What would life be like if Earth did not undergo hurricanes? Scientific evidence suggests that Earth's rotation speed probably has the greatest effect on the number and intensity of storms the planet generates each year.2 If its rate were to change by as little as two hours per day, slowing from 24 to 26 hours, the number of violent storms, including thunderstorms and hurricanes, would certainly decrease. (On the other hand, a faster rotation rate would result in more numerous and far more devastating storms.) Perhaps hurricanes might disappear altogether; so humans would live in a much more benign environment-or would they? There is evidence that a planet without hurricanes, as devastating as they are, may not represent an improvement. Earth derives a number of benefits from massive thunderstorms (of which hurricanes are the most severe), including these five:3 1. 2. 3. 4. 5. Sufficient rainfall to water the earth. Major parts of the world rely on heavy storms to supply water for life's basic needs. Plant fertilizer from lightning. Nitrogen "fixing" by lightning converts some of the nitrogen in the air into a form that plants can use for food. Without it, many plants could not thrive. And plants are the foundation of humanity's food chain. Pruning of forests and prairies from lightning fires. Fires help maintain the diverse life-forms needed for a stable ecology naturally, by clearing away old growth and spurring new plant growth required for food. Pruning of forests by strong winds. In addition to fires, winds uproot weaker trees and open up the forest canopy for a greater diversity of plants and animals. Drought-breaking rainfall. Severe storms such as hurricanes (called monsoons, typhoons, or cyclones in other parts of the world) provide immediate, ample water supplies to end years of drought. Earth's rotation speed is fast enough to provide the just-right quantity and magnitude of thunderstorms to sustain a rich diversity of life. But with that provision come occasional hurricanes in certain areas, storms with locally tragic effects. Rather than charging God with poor design or asserting that He does not exist or care, perhaps the best response would be to research and supply the ways and means to better protect people living in hurricane-prone regions. (Check out the newspaper article written by RTB apologist Mark Ritter for some additional thoughts.4) References 1. 2. 3. 4. See Ronald H. Nash, Faith & Reason (Grand Rapids, MI: Zondervan, 1988), 177-221; Kenneth Richard Samples, Without a Doubt (Grand Rapids, MI: Baker, 2004), 239-53; Hugh Ross, The Creator and the Cosmos, 3rd ed. (Colorado Springs, CO: NavPress, 2001), 175-99; and Krista Kay Bontrager, "Good God, Cruel World?" http://www.reasons.org/resources/skeptics/goodgod.shtml, accessed 10/27/05. A. Navarra and G. Boccaletti, "Numerical general circulation experiments of sensitivity to Earth rotation rate," Climate Dynamics 19 (2002): 467-83. Chuck Doswell, "Is there a good side to severe storms?" http://webserv.chatsystems.com/~doswell/goodwx.html, accessed 10/27/05. Mark Ritter, "Maybe there's some good in those 'canes," North County Times, October 2005, accessed 11/15/05. Subjects: Earth/Moon Design, Natural Disasters Designed to Shake April 1st, 2007 By Administrator 4/1/2007 by Dr. Hugh Ross My family lives in one of the fastest-rising neighborhoods in the nation—not economically, but topographically. Our home rises by an average of 9 millimeters (1/3 inch) per year. Sometimes the elevation gain (via earthquake) seems a bit disturbing. Sometimes it's destructive. Nonetheless, I tell my wife and sons we should be thankful for all the uplift we get. Specifically, we can thank God for designing Earth for vigorous and virtually constant plate tectonic activity. Why? Because such movement is essential for life. Earth has experienced robust plate tectonics for four billion years. Without it, our planet would possess no continents, no mountains, no stable water cycle, and nothing like the diversity and abundance of life we enjoy.1 In fact, without tectonic activity, Earth would have no mechanism to compensate for ongoing changes in the Sun's luminosity, and all life would be driven to extinction.2 Without such large-scale motions, nutrientrestoring cycles would fail to provide for life's basic needs3 and humanity would lack the abundant biodeposits (like coal, oil, natural gas) on which civilization depends.4 For some time now scientists have recognized the importance of plate tectonics, but only recently have they discovered the degree to which Earth's tectonics reflect exquisite fine-tuning. This understanding was greatly enhanced when two planetary physicists, Diana Valencia and Richard O'Connell at Harvard University, developed detailed models of the internal structure of massive rocky planets.5 Their research showed that as the mass of a rocky planet increases, the thickness of its crustal plates decreases, and so does its resistance to tectonic motion. Therefore, the greater the mass of a rocky planet, the higher the probability for plate tectonic activity and the more aggressive that activity will be. Valencia and O'Connell's study helps explain a solar system enigma—why only Earth, of all the planets in our solar system, manifests plate tectonics. Liquid water is the key. If it weren't for Earth's abundant surface water, its crust wouldn't crack and move. Water lowers the yield strength of certain crustal minerals. For example, water cuts in half the yield strength (resistance to crumbling) of olivine, a primary constituent of Earth's crust. For permanent, strong plate tectonics to be possible on a dry rocky planet, the planet's mass would have to be more than twice that of Earth. (At such a mass, the planet approaches the boundary between rocky planets and gaseous planets.) And even though the presence of liquid water lowers the mass boundary for strong and ongoing tectonics, Earth's mass represents the lower limit, according to the Harvard team's calculations. (Previous studies put the plate tectonics limit at one-third the mass of Earth, but such a low mass allows only for weak or ephemeral plate tectonic activity.) This finding becomes especially remarkable in light of the fact that from a physiological perspective, Earth's mass could not be any larger and still be suitable for life (specifically for respiration). The problem for a planet more massive than Earth is its atmosphere. The more massive a planet, the thicker the atmosphere it accumulates during its formation. And, the atmospheric thickness rises geometrically with a planet's mass. For example, Venus, with seven times the mass of Mars, has an atmosphere more than 600 times thicker. In fact, Earth's atmosphere would be too thick for breathing if it weren't for its low-velocity collision with a Mars-sized object early in its history, a collision that blew away most of the thickness.6 As Valencia and O'Connell's research points out, a planet's mass must be virtually identical to Earth's for that planet to have a chance at sufficient-for-life tectonics. It also must be as wet as Earth but no wetter. (A wetter planet would lack continents and critical nutrient cycles.) It seems the more researchers learn about planets, the more evidence they find for the purposeful shaping of Earth for life. References: 1 Hugh Ross, Creation as Science (Colorado Springs: NavPress, 2006), 129-38. 2 Ibid., 129-36. 3 Hugh Ross, The Creator and the Cosmos, 3rd ed. (Colorado Springs: NavPress, 2001), 187-99 4 Ross, Creation as Science, 128-29, 140-41. 5 Diana Valencia and Richard J. O'Connell, "Inevitability of Plate Tectonics on Super-Earths," Astrophysical Journal Letters 670 (November 20, 2007): L45-L48. 6 Robin M. Canup, "Simulations of a Late Lunar-Forming Impact," Icarus 168 (April, 2004): 433-56; Robin M. Canup, "Dynamics of Lunar Formation," Annual Review of Astronomy and Astrophysics, vol. 42 (Palo Alto, CA: Annual Reviews, 2004), 441-75; M. Touboul et al., "Late Formation and Prolonged Differentiation of the Moon Inferred from W Isotopes in Lunar Metals," Nature 450 (December 20, 2007): 1206-9; Kaveh Pahlevan and David J. Stevenson, "Equilibration in the Aftermath of the Lunar-Forming Giant Impact," Earth and Planetary Science Letters 262 (October 30, 2007): 438-49; T. Kleine et al., "Dating the Giant Moon-Forming Impact and the End of Earth's Accretion," American Geophysical Union Meeting 2005, abstract #P41E-04 (December, 2005). Subjects: Biodeposits, Earth/Moon Design, Extrasolar Planets, Faint Sun Paradox, Life on Other Planets, Plate Tectonics, TCM - Faint Sun Paradox A Carbon-14 Coincidence March 7th, 2008 By Dr. David Rogstad Why does the Moon have almost exactly the same apparent size in the sky as the Sun, so that it perfectly blocks out the Sun in a solar eclipse? A few weeks back I mentioned the benefits to scientific discovery provided by this coincidence. By using it, astronomers learned about the Sun’s corona, were able to test Einstein’s general theory of relativity, and determined the past rotation rate of the Earth, all at a time earlier than would have been otherwise impossible. While there is no scientific reason for this remarkable coincidence, it suggests that God may have provided it as a tool so that we could more quickly discover characteristics of His creation. Another coincidence has provided archeologists and paleontologists with an extraordinary tool for dating objects that contain carbonaceous material. I speak of carbon-14 with its just-right-for-dating half-life of 5,730 years. This coincidence is remarkable because C-14, along with the inert C-12, not only is an important component of fossils, but if C-14 had a longer half-life, it wouldn’tt provide the accuracy for more recent fossils; if shorter, it wouldn’t work for longer time periods. In a paper published in the latest issue of Physical Review Letters (available in preprint here and as a news summary here), Jeremy Holt and colleagues note in their introduction that C-14 was not expected to have a long half-life. Based on previous models, physicists expected it to have a similar half-life to that of C-11 at 20 minutes, or of oxygen-14 at 1 minute, or O-15 at 2 minutes, or that of nitrogen-13 at 10 minutes. Why does C14 have a half-life of 3 billion minutes? This has been a mystery to theorists for half a century. However, in a breakthrough discovery, Holt and his collaborators have performed a new calculation that includes the change of the meson mass as it travels through an atomic nucleus. Mesons are atomic particles that are believed to mediate the force between two nuclei, and play a role in the radioactive process taking place in C-14. From these calculations the authors were able to account for why C-14, but not the other nuclei, has this long radioactive half-life. Radiocarbon dating is a method that depends on the naturally occurring presence of C-14 in the material to be dated. Every living thing constantly exchanges C-14 with its environment as long as it lives. Once it dies, the exchange stops. Consequently, scientists assume that a fossil’s C-14 content does not change once the sample to be dated ceases to incorporate carbon, except for the amount gradually depleted radioactively with its 5,730-year half-life. If the amount present when its activity ceased can be determined, then the ratio of the initial amount to the existing amount is related to how long ago it was alive. While scientists must account for misuse of this technique and use proper calibration, in most cases it can provide a very accurate way to date many fossil samples containing carbon. Radiocarbon dating is an important tool that has yielded rich insight into the events and processes in Earth’s history. Because many of the key truth-claims of the Bible are rooted in history, this technique has also provided support for their veracity. Subjects: Laws of Physics, Radiometric Dating Techniques, Universe Design Some Like It Hot—Especially the Continents August 15th, 2007 By Dr. Jeff Zweerink I ran across an interesting article in Science Daily a few weeks ago. The article’s authors point to evidence that the vast majority of North America would reside under water if the rocks making up the continental crust were cooled to the same temperature as some of the oldest crust underneath Canada. While it is not surprising that the oceans might cover coastal cities of New York, Miami, New Orleans, and Los Angeles, water hundreds of feet deep would drown even mountain cities such as Denver and Salt Lake City. Clearly plate tectonics plays a critical role in the formation and sustaining of continental crust. However, the temperature of the rocks figures just as importantly in ensuring that continents remain above sea level. Adding heat to rock makes it expand and, therefore, become less dense. Less-dense rock floats higher above the denser surrounding crust, meaning the surface rock sits at higher elevations. In fact, the authors of the original scientific article note that “temperature differences within the Earth’s crust and upper mantle explain about half of the elevation of any given place in North America” (emphasis added). The bulk of life’s diversity on Earth is found on the land and shallow seas of the continental crust. Less continental crust provides fewer habitats for life and also limits the capacity of plate tectonics to regulate the global temperature. (Peter D. Ward and Donald Brownlee’s book Rare Earth highlights the importance of plate tectonics in maintaining a global temperature supportive of liquid water oceans.) While studies for other continents remain to be done, we expect similar conclusions since North American crust exceeds the average density of continental crust across the globe. The heat content of Earth’s continents appears to be finely tuned to ensure Earth’s habitability for human life. Subjects: Earth/Moon Design, Geophysical Design Petroleum: God's Well-Timed Gift to Mankind September 1st, 2004 By Dr. Hugh Ross I am old enough to remember the days when gasoline sold for $.26 a gallon. But, even at today's high prices, gasoline is a bargain compared to what it could cost if it were not so easily and abundantly accessible. Recent research by geologists and physicists reveals that humans are living at the best possible time in Earth's history for harvesting petroleum-a resource that helped launch and sustain advanced civilization. Without a series of just-right geophysical events and conditions, there would be no complaining about pump prices, because there would be little or no fossil fuel to complain about. To appreciate this miracle of fuel's availability to humanity one needs to understand how petroleum forms and is stored in the earth. First, sedimentation and plate tectonics bury organic material. This buried organic matter is transformed by heat, pressure, and time into kerogen (high-molecular-weight tars). With yet more time and heat a significant portion of the kerogen is converted into petroleum. 1 Through still more time, however, microbial activity works to degrade petroleum into methane (natural gas). 2 Certain kinds of organisms are much more likely upon death and burial to be transformed into kerogen than others. The most efficient kerogen producers were the swarms of small-body-size animals that inhabited large shallow seas soon after the Cambrian explosion (so named because 50-80% of animal phyla "exploded" onto the scene 543 million years ago). If the Creator's goal is to provide humanity with the richest possible reserves of fossil hydrocarbons, a fixed period of time must transpire between the epoch when efficient kerogen producers were dominant on Earth (about 500 mya) and the appearance of human beings (some tens of thousands of years ago). With too little time, not enough petroleum will be produced. With too much time, too much of the petroleum will be degraded into methane. There is more to the production of fossil hydrocarbon reserves than just the burial of particular organisms and their progressive conversion into kerogens and petroleum. Certain sedimentation processes are needed to lay down the porous rocks that will become reservoirs. Later, these rocks must be overlaid with fine-grained rock with low permeability (sealer rocks). Finally, certain tectonic forces cause appropriate caps under which fossil hydrocarbons can collect.3 Long years of specific sedimentary and tectonic processes are required to produce appropriate reservoir structures for collecting and storing fossil hydrocarbons. And yet too much time will lead to the destruction of the reservoirs. Additional tectonic and erosion processes eventually cause the reservoirs to leak. If too much time had transpired before humans came on the scene the fossil hydrocarbon reservoirs would have emptied, and the resources with which human beings were able to launch an industrial and scientific revolution would have been missing or insufficient. Both methane and kerogen play significant roles in sustaining modern civilization and technology, but their importance pales in comparison to petroleum, particularly in the plastics industries. While human technology is now sufficiently advanced to consider and develop ways to get by without petroleum, it seems doubtful that such technology would have arisen without access to large amounts of petroleum to begin with. Human beings indeed arrived at the optimal "fossil-hydrocarbon moment." Such optimized timing raises reasonable doubt about any naturalistic model for life and humanity, but aligns perfectly with what a biblical creation model would predict. Subjects: Biodeposits, Earth/Moon Design, Geophysical Design, Life Design, TCM - Life Design Bacterial Design for Recycling Phosphorous March 20th, 2005 By Dr. Hugh Ross A microbiologist and a geologist in Germany have found some amazing design features in a large sulfur bacterial species that benefits all life. Thiomargarita namibiensis is a colossal bacterium (nearly 1 mm in diameter) that thrives in surface marine sediments under both oxic (containing oxygen) and anoxic conditions. It periodically contacts oxic bottom water to take up nitrate. Such internally stored nitrate allows it to survive for long periods under anoxic conditions. The bacterium’s prime energy source is sulfide oxidation. The sulfide accumulates in anoxic marine sediments when sulfate-reducing bacteria there degrade organic matter. The researchers discovered that aggressive sulfide oxidation by large populations of T. namibiensis is responsible for phosphorite deposits in marine sediments. Such deposits play a critical role in the life-essential phosphorous cycle. The amazing, unique designs and behaviors of T. namibiensis that allow it to take advantage of sulfide produced by sulfate-reducing bacteria so as to sustain Earth’s phosphorous cycle at an ideal rate for the benefit of all life testifies of a supernatural, super-intelligent Creator. Heide N. Schulz and Horst D. Schulz, “Large Sulfur Bacteria and the Formation of Phosphorite,” Science 307 (2005): 416-18. Related Resource o Hugh Ross, “Anthropic Principle: A Precise Plan for Humanity” Product Spotlight o Journey Toward Creation, 2nd ed., by Hugh Ross GOE or Die: Earth’s Habitability No Sure Thing September 1st, 2009 By Dr. Jeff Zweerink Jason Bourne lives life on the edge. The protagonist of the Bourne spy fiction thriller series relentlessly pursues the truth, with danger lurking at every turn. Regardless of the peril, he must continue. In ways, Earth’s history demonstrates similar hazards. Starting from the most inhospitable circumstances, numerous physical transformations now enable Earth to teem with life. But many of those events brought the Earth—and its life–– to the brink of extermination forever. One such change occurred roughly two-and-a-half billion years ago. For the first two billion years, Earth’s atmosphere contained no free (uncombined with other elements) oxygen. Although oxygen was present, it was tied up in molecules like carbon dioxide and water vapor. Yet anything more sophisticated than single-celled organisms requires free oxygen because of the energy oxygen releases during chemical reactions. Fortunately, photosynthetic organisms appeared on the scene at this time and began producing abundant quantities of oxygen. Over the course of a couple hundred million years, these organisms delivered a permanent oxygen component to Earth’s atmosphere—although at levels much lower than today. Scientists refer to this period as the Great Oxygenation Event (GOE). On the positive side, the permanent oxygen component generated an ozone layer in the stratosphere. Since the GOE, the ozone layer has protected life from the harmful ultraviolet radiation emitted by the Sun. Negatively, this permanent oxygen reservoir also began wreaking havoc with Earth’s temperature. The dominant greenhouse gas before the GOE was methane (CH4). (While carbon dioxide receives a lot of press as a greenhouse gas, methane is over 60 times more effective at trapping heat from the Sun.) Because of oxygen’s high reactivity, during the GOE it reacted with the methane to produce carbon dioxide (CO2) and water (H2O). Like turning off a thermostat in the middle of winter, Earth’s temperature would have plummeted. In fact, an international team of geologists recently discovered evidence for extensive glaciations corresponding to this increase in atmospheric oxygen.1 This was the first widespread ice age on Earth. The dramatic nature of this cooling likely resulted in glaciers covering the entire surface of Earth. Such a state, if it persisted, would drive life to near extermination. However, it appears the same process that initiated the covering of Earth with glaciers also helped remove the glaciers. Cooler oceans dissolve more oxygen. This dissolved oxygen then reacted with the carbon remains of previous life that rested on the ocean floor. The abundant carbon dioxide released further enhanced the greenhouse heating, leading to a warmer Earth. By itself, either event (the cooling induced by the GOE or warming caused by the reaction of dissolved oxygen with carbon remains) had the potential to render Earth uninhabitable. The fact that both occurred concurrently suggests that a supernatural Designer––like a spy thriller novelist who knows where the plot’s going–– orchestrated both events in order to prepare Earth for the arrival of human beings. Reference: 1 Qingjun Guo et al., “Reconstructing Earth’s Surface Oxidation across the Archean-Proterozoic Transition,” Geology 37 (May 2009): 399–402. Subjects: Geophysical Design The Measurability of the Universe––a Record of the Creator’s Design October 1st, 2000 By Guest Author by Dr. Guillermo Gonzalez If the universe were not measurable, scientific study would be impossible. Astronomy, biology, chemistry, cosmology, geology, physics, and the other disciplines of science would be no less quixotic than alchemy or astrology. Science would not—could not—shed much light in the cosmic darkness. Most scientists take the measurability of the physical realm completely for granted: It is measurable because scientists have found ways to measure it. Scientists (myself included) may take pride in our ability to make measurements––especially those measurements requiring ingenuity, persistence, and skill––but why take the universe’s measurability for granted? Is there any deep significance to the measurability of the universe? The answer springs from the very foundations of science, from the philosophical assumptions (chiefly drawn from the Judeo-Christian Scriptures1) on which scientific endeavor rests. These assumptions include, among others, the existence of a theory-independent external world, the existence of order in the external world, the reality of truth, the validity and reliability of the laws of logic and mathematics, the basic reliability of sense perception, and the adequacy of the human mind to comprehend the universe. 2 The Judeo-Christian vision of reality predicts a unique correspondence between the physical universe and the human mind. By identifying the aspects of measurability humans cannot influence or control, one can determine (at least roughly) whether or not the measurability of the universe requires supernatural fine-tuning, and if so, to what degree. This study begins with a look at the nearby cosmos and from there moves outward in space, backward in time. The Measurability of the Earth One of the characteristics that makes Earth such an ideal “recording device” is its built-in set of time markers– –cyclical rhythms on time scales of days, months, seasons, years, centuries, periods, eras, and eons. Humanity could have found itself in a far less measurable place. The Moon, for example, does not have active weather, seasons, or tectonics, and therefore offers few time markers. The Moon looks ancient, yet ageless. Jupiter and the other gas giants have active weather, but they lack any solid surface on which to record their rhythms and events. The thin crust of the Earth provides not only a safe and comfortable place for living creatures of all kinds, but it also serves as the planet’s information storage space. The deep, hot interior of the planet, the atmosphere, and the oceans are all too fluid to preserve much of the past. Earth’s cycles provide the steady beat of time markers, with other, more subtle, fluctuations superimposed. Because of seasonal changes in weather and plant life in a given locale, growth and deposition phenomena leave easily distinguishable (and measurable) features. Growth rings in trees not only yield information on the rain and temperature for a given season, but they also provide a unique tool for measuring the carbon-14 content of the atmosphere, which is modulated, in turn, by the sunspot cycle. Research on tree rings gives astronomers information about solar variations on a wide range of time scales, from decades to millennia. Snow deposits in Greenland and Antarctica have created a four hundred-thousand-year record of the composition of Earth’s atmosphere. 3 Ancient air bubbles trapped within these deposits allow us to measure the concentration of carbon dioxide and other gases in past eras. The snow deposits give us a measure of ancient dust levels, which are indicative of large volcanic eruptions or very dry conditions. They also enable us to measure the ratios of three oxygen isotopes, which indicate the mean global temperature in past epochs. According to a very recent study, nitrate spikes in Antarctic ice deposits may help us trace supernova events (gigantic star explosions) of the past thousand years. Certain features of the ocean floor allow us an even longer-range view, hundreds of millions of years back into Earth’s history. At the mid-ocean ridges (“spreading centers”), new sea floor is produced when molten rock upwells from the hot mantle below. When the molten rock solidifies it records the state of the earth’s magnetic field at that time. By studying these sea-floor records at varying distances from the spreading centers, oceanographers can “read” the history of fluctuations in Earth’s magnetic field. A phenomenon so subtle as to be unnoticeable in everyday life is reliably recorded and preserved for later discovery and deciphering. Ancient “tidalites” (tidal sediment layers) and coral, mollusk, and stromatolite growth layers record the lunar and solar tidal cycles, giving us unique data on the length of terrestrial days and lunar months in ancient times. Such data tell us that 500 million years ago, a day was about 20 hours long and a month was about 27.5 (present-epoch) days.4 Meteorites that have hit the earth provide another treasure trove of data (preserved for billions of years) waiting to be unlocked. Many meteorites come from the asteroid belt, where collisions between asteroids send shards hurtling throughout the inner solar system (planets from Mars inward) and occasionally to the earth. Fragments falling on the ice fields of Antarctica are the best preserved ones, and their dark appearance makes them easy to distinguish against the uniform blue-white background. Today, a meteorite’s individual grains, each measuring less than a millimeter in width, can be separately analyzed. These grains yield invaluable clues to the sources of short-lived (now extinct) “radionuclides” present in the gas-and-dust cloud from which our sun and solar system formed. They also give us clues to the timing of certain key events in the formation of neighboring planets. Even more amazing is the discovery that meteorites carry what appear to be individual interstellar dust grains, each from a different star that existed before the Sun. These dust particles give us rare and important data on the chemical history of the Milky Way. It appears that as part of God’s grand design of the cosmos, He has provided a method of collecting, preserving, and delivering to our doorstep tiny bits of distant (both in the spatial and temporal sense) stars. What more could an astronomer ask for? On a less grand scale, small bits of the moon and Mars have been blasted to the earth by large impacts. The most famous of these is the Martian meteorite, ALH 84001 that stirred much media attention a few years ago. The Moon probably contains a rich reserve of unaltered planet shards from the early history of the solar system. One might think of the Moon as the earth’s attic, where ancient artifacts are stored and forgotten, perhaps to be retrieved one day. The Measurability of the Sun Total eclipses of the Sun as seen from the surface of the earth may be described as both “useful” and “exceptional.”5 Apart from the deep awe they inspire in every people group from remote tribes to astrophysicists, these eclipses allow us to study the Sun’s corona, test general relativity, and calculate the slowdown of the earth’s rotation. They are exceptional in that they are nearly “perfect;” that is, the earth and Moon are similar in size, the solar and lunar profiles on the sky are nearly perfect circles, and the Sun appears to be larger when it is viewed from Earth than when it is viewed from any other planet with moons. The likelihood of finding this combination of features is remote. Of the roughly 65 natural satellites (moons) in the solar system, none even comes close to producing such clear and spectacular eclipses. What’s more, humans live at a special time with respect to the observability of total solar eclipses. Since the Moon is spiraling away from Earth and the Sun is swelling due to its changing internal structure, such eclipses are possible only for a relatively brief time span. They will continue only for about 250 million years. That may seem like a long time, but it constitutes only approximately 5% of Earth’s history. The Sun’s radiation conveys a wealth of information. By observing its spectrum, researchers learn about the Sun’s composition, surface temperature, and surface gravity. This “readable” spectrum is not unique to the Sun, but the Sun’s spectrum is nearly optimal in terms of measurability and the number (and abundances) of chemical elements it reveals. This optimal quality of the Sun’s measurability derives from characteristics other than its proximity to Earth and the large number of photons arriving at Earth-based instruments. In comparison to the spectra of other stars with similar “signal-to-noise ratio” (data quality), the Sun’s spectrum contains more extractable information. The Sun’s particular surface temperature and its relatively low luminosity allow for the extraction of more information. The remarkable convergence of these just-right characteristics maximizes its readability. The Astronomical Realm The light sent to Earth from sources outside the solar system contains a wealth of information about stars, nebulae, galaxies, and even the intervening matter. Using various techniques and instruments, astronomers have used that light to map out most of the Milky Way disk, clearly delineating its spiral arm structure. The measurement of the three-dimensional space motions of stars in the Milky Way is possible only because stars can be treated as if they were mathematical points. This feature allows astronomers to measure the relative positions of stars very precisely, and it means that stars can be used as simple probes of the Milky Way’s gravitational field. If stars were larger and the distances between them smaller––like nebulae, for example––then the mathematics would be much more complex. Stars’ positions and other features would be far less measurable, because their light would be spread over a larger volume of space. Also, if the Milky Way contained fewer stars, it would yield fewer and more obscure clues about its history and structure. Astronomers have discovered that certain light sources are particularly useful as “standard candles” (see sidebar). Examples of standard candles are Cepheid and RR Lyrae variable stars. The pulsation period of a Cepheid variable is related to its intrinsic luminosity in a simple way. By measuring the period and mean apparent brightness of a particular Cepheid variable star, one can easily calculate its distance. Because of the simplicity and consistency with which these objects operate, they provide invaluable reference points, or units of measure. Astronomers rely on this important data to reveal some of the fundamental constants of the universe. The cosmic microwave background radiation, first detected in 1965, has enabled cosmologists to extract information on enormous size- and time-scales. With the launch of the Cosmic Background Explorer (COBE) satellite in 1989, astronomers were able to make measurements precise enough to confirm several predictions of the Big Bang theory (a theory consistent with the Bible) and effectively kill both the Steady State hypothesis and the oscillating universe hypothesis. Atheistic cosmologists as a way to avoid a beginning for the universe had favored these hypotheses. Two upcoming space missions, the NASA Microwave Anisotropy Probe (MAP) and the European Space Agency (ESA) Planck Surveyor, promise orders of magnitude improvement over the measurements the pioneering COBE satellite recorded. The background radiation is sufficiently intense that we can measure it precisely with modern instruments, but not so strong that it is unaffected by processes shortly following its creation. Therefore, we can learn about certain parameters of the universe at very early times, constrain some aspects of fundamental physics, and garner a glimpse at early large-scale structure and formation. As the universe ages, the background radiation will become less measurable. First, the continued expansion of space-time will cause it to become less intense and more redshifted. Second, as stars continue to form in the Milky Way, they will contribute to greater foreground contamination, resulting in greater difficulty in measuring the ever-fading background. Teleological Implications In terms of its mass, the Sun is among the top 10% most massive stars in the solar neighborhood. 6 Aside from obvious questions of habitability, what if humans were attempting to scan the skies from a planet orbiting one of the less massive stars, one of those among the 90% majority? What would they be able to detect and measure? The most fundamental ruler in their astronomical “tool chest” would be less effective. It is the method called stellar parallax. Earth’s inhabitants can use the changing position of the earth in its orbit around the Sun to detect the apparent reflex motion of nearby stars relative to distant background stars. By this method they can measure the distance from the earth to those nearer stars. M dwarfs are the most common type of star in the Milky Way. The habitable zone comprises the place around a star where liquid water can exist on the surface of a terrestrial-like planet continuously. The estimated diameter of the habitable zone around an M dwarf is only about 10% that of the zone around the Sun, the zone in which Earth resides. Therefore, for a planet orbiting an M dwarf, the effectiveness of the stellar parallax method would be severely diminished. In fact, astronomers on such a planet would be able to observe only one-thousandth the volume of space Earth-bound astronomers can observe. The distances to many rare types of stars, such as O and B stars, and Cepheid and RR Lyrae variables, would remain a mystery, and information they provide would be inaccessible. Clearly, M dwarfs would be less hospitable for life, and the cosmos far less measurable from their environs. Since measurability is not a requirement for habitability, one cannot invoke the Anthropic Principle 7 to make the remarkable measurability of the universe seem less remarkable. Evidence suggests that the universe was designed not only for human habitability but also for human measurability and comprehensibility. The same processes and features that make Earth habitable also make and preserve a record of activity and provide a means for measurement. Those very places in the Milky Way that would be most dangerous to humans (e. g., the galactic center, globular clusters, and spiral arms) also offer the poorest visibility and opportunity to make measurements. Does it seem a mere coincidence that Earth’s location in the Milky Way affords an optimal view of most of the universe? Humanity’s home planet is a comfortable porch from which curious humans can gaze out to the ends of time and space. This argument allows us to ascribe purpose to any fine-tuned, measurable aspect of the universe, such as stars and galaxies, earthquakes, neutrinos, and the Moon. If anyone asks, “Why are there so many stars and galaxies in the universe?” One can respond with double impact: Not only is a universe as big as this one required for any kind of life, but only a vast number of stars and galaxies permits intelligent creatures to measure (reliably) the basic parameters of the universe. Earthquakes are important not only because life needs the effects of plate tectonics but also because they allow us to probe the internal structure of the Earth, which could not be done any other conceivable way. Neutrinos give us a way to measure the temperature of the sun’s core and to study the details of neutron star formation in supernovae explosions. The Moon records some of the early history of the solar system and takes part in producing wonderful eclipses. And so on. Of course, this consideration brings us to the deeper, theological question: Why would the Creator make the universe so measurable? What’s the point of allowing humans to measure the characteristics of the universe? To those who hold a Christian worldview, the answer is clear. In fact, the Bible explicitly states it: “For since the creation of the world God’s invisible qualities, His eternal power and divine nature, have been clearly seen, being understood from what has been made, so that men are without excuse” (Romans 1:19-20). Sidebar: Standard Candles Astronomers employ some types of stars as “standard candles.” These are stars that have luminosities that are in some way standard. As a simple everyday example of a standard candle, consider an ordinary 100-watt light bulb. Because a light bulb has a constant luminosity (or intrinsic brightness) we can estimate its distance from us if we can measure its apparent brightness. This technique only works if we have good reason to believe the luminosity of a given light source is some standard value. For a distant light bulb, one can verify its luminosity by observing it with a telescope and looking for the phrase “100 watts.” Of course, this does not work with stars, but the principle is similar. References: 1. 2. 3. 4. 5. 6. 7. See Stanley L. Jaki, The Savior of Science (Washington, D.C.: Regnery Gateway, 1988). J. P. Moreland, The Creation Hypothesis (Downers Grove: InterVarsity Press, 1994), 17. J. R. Petit, et al., “Climate and Atmospheric History of the Past 420,000 Years from the Vostok Ice Core, Antarctica,” Nature 399(1999): 429-36. C.P. Sonett and M.A. Chan, “Neoproterozoic Earth-Moon Dynamics: Rework of the 900 Ma Big Cottonwood Canyon Tidal Laminae,” Geophysical Research Letters 25 (1998): 539-42. Guillermo Gonzalez, "Wonderful Eclipses," Astronomy & Geophysics, (June 1999): 3.18-3.20. Guillermo Gonzalez, “Is the Sun Anomalous?” Astronomy & Geophysics 40 (October 1999): no. 5, 2529. Hugh Ross, The Creator and the Cosmos 2d ed. (Colorado Springs, CO: Navpress, 1995), 92, 121-25, 128. Subjects: Atheism, Constants of Physics, Earth/Moon Design, Einstein / Relativity, Galaxy Design, Geophysical Design, Laws of Physics, Solar System Design, TCM - Cosmic Design Vital Poisons July 1st, 1999 By Phil Chien 7/1/1999 by Dr. Hugh Ross Perhaps you have noticed the addition of Food and Drug Administration warnings to packages of dietary supplements sold in drug and health food stores. If not, please do. These warning labels subtly announce dramatic new evidence for the divine design of life—and of the earth for sustaining life. Research has identified many dietary essentials, in addition to the familiar one, iron, to be harmful, if not deadly, in certain amounts. Such elements as chromium, molybdenum, selenium, and vanadium, for example, are essential for building proteins, and proteins serve as life’s molecular “factories.” Yet each of these elements is toxic in any but the “just right” amount. A finely-tuned balance of such elements in organisms’ external environment also proves necessary but risky. Molybdenum, for instance, though it can be harmful plays a crucial and unique role in “nitrogen fixation,” the process by which nitrogen from the atmosphere attaches to chemicals that can be assimilated by plants. This particular process, without which land life cannot exist, is impossible unless a certain “right amount” of molybdenum resides in the soil. For many years, we have recognized the devastating effects of iron deficiency or iron overabundance in the diet of humans and advanced animals. Year by year, however, the list of lethal yet essential substances grows. Currently that list includes arsenic, boron, chlorine, cobalt, copper, fluorine, iodine, manganese, nickel, phosphorus, potassium, sulfur, tin, and zinc, in addition to the four mentioned above.1 At the same time, our astronomy research reveals that the earth’s crust differs significantly from the crusts of other solar system bodies. One difference lies in the relative abundance of various life-essential elements. Earth’s crust contains “just right” quantities of all the elements necessary for the existence and sustenance of advanced land life. This finding can be viewed as a remarkable (more accurately, an impossible) coincidence or as a wondrous indicator of design. To reach for a sound bite, I would say that the gastronomical and astronomical evidences favor purposeful planning and preparation. Reference: 1. John Emsley, The Elements , third edition (Oxford, UK: Clarendon Press, 1998), pp. 24, 40, 56, 58, 60, 62, 78, 102, 106, 122, 130, 138, 152, 160, 188, 198, 214, 222, 230. Subjects: Geophysical Design, Life Design, Speciation Events Earth’s Ancient Magnetic Field March 24th, 2010 By Dr. Jeff Zweerink Not only are magnets fun to play with, they play prominent roles in humanity’s just-right habitat. Magnetic fields affect the way stars and galaxies form, provide navigational landmarks for human and animal travel, and ensure Earth retains its water for more than four billion years. But has Earth’s magnetic field existed for most of the planet’s history? Recent research addresses this question. The main difficulty of studying Earth’s ancient magnetic field is finding materials that record its strength. Usually, geophysicists use long-solidified crustal rocks. The magnetic field affects the formation of certain kinds of rocks in such a way that allows scientists to determine the field’s strength and direction. While finding these ancient rocks is difficult enough, other factors impact researchers’ ability to extract information from them. For example, any significant heating of the rocks removes the original information recorded about the magnetic field. Also, researchers must keep in mind that a steady stream of charged particles impacts the top of Earth’s atmosphere, inducing a magnetic field. In order to determine the characteristics of the magnetic field generated by the planet core, this atmospheric contribution must be accounted for. A team of international scientists recentlydeveloped a technique to carry out the necessary measurements.1 They started with dacite rocks found in South Africa dating from 3.4 to 3.45 billion years ago. These rocks contain magnetic inclusions that record information about Earth’s core-generated magnetic field (along with atmospheric magnetic noise) from this time. By carefully selecting inclusions meeting specific criteria, they isolated a pristine sample of small quartz crystals to analyze for magnetic field information. Using a SQUID magnetometer, the scientists determined that 3.4 to 3.45 billion years ago Earth’s magnetic field was 50 to 70 percent of today’s values. Three aspects of these results warrant further note. First, this measurement provides the earliest measurement of Earth’s magnetic field—besting the previous value by 200 million years. Second, the field strength measured here indicates that scientists are finding evidence from near the time when Earth’s rotation-driven dynamo first started gaining significance. If so, even more ancient measurements will provide important insights into the process of core formation and dynamo generation. Third, and perhaps most important, these measurements identify a critical period for maintaining habitability on Earth for the next 3.5 billion years. A smaller magnetic field means that the solar wind interacts more directly with Earth’s atmosphere. The key interaction consists of radiation breaking water into hydrogen and oxygen and the hydrogen escaping into space. This reaction results in loss of water. If our planet had experienced a stronger solar wind and weaker magnetic field for the first billion years of Earth’s history, water would have been rapidly stripped from the early Earth. This situation may explain why Earth has the just-right amount of water today (and not as much as models predict). As crucial as water is to life, it must come in the right amounts. This research of quartz crystals from South Africa adds to a growing body of evidence that multiple, diverse processes (astronomical, geophysical, atmospheric, and biological) must all interact precisely to ensure Earth has the right amount of water. Such finely-tuned interactions point to a divine Designer. Endnotes: 1. John A. Tarduno et al., “Geodynamo, Solar Wind and Magnetopause 3.4 to 3.45 Billion Years Ago,” Science 327 (March 5, 2010): 1238–40 Subjects: Earth/Moon Design Why Does the Earth have Oceans? November 18th, 2009 By Dr. Jeff Zweerink A few weeks ago, I posted a TNRTB describing the special circumstances in Earth's early history that ensured we had an adequate supply of osmium and iridium with which to develop a technological civilization. Further developments indicate that the carefully orchestrated events on early Earth played an even more critical role in establishing this planet's habitability. Without those events, Earth would not enjoy an abundance of liquid water or the life-essential plate tectonics it facilitates. Consider this brief description of how the solar system formed. A gas cloud began collapsing, resulting in a disk of material around the protosun at the center of the cloud. As the particles in the cloud interacted, they grew in size. At some point, these particles began to gravitationally attract other material from the disk until they became planet-sized. The last step of planet formation occurred when these planet-sized objects merged to form Mercury, Venus, Earth, and Mars. The gas giants formed in a similar fashion except they grew rapidly enough to gravitationally attract a sizable fraction of hydrogen and helium before the solar wind blew these gases out of the solar system. Until recently, scientists believed that the oceans that currently cover Earth (similar to the water that once covered Mars and Venus) arose when water in the formation material escaped to the surface. However, a growing body of evidence now indicates that these Earth-forming materials did not contain enough water to form the oceans. This is because Earth resides inside the snowline, locations closer to the Sun than the asteroid belt. Inside the snowline (indicated by the red region in the image-link below) high temperatures kept any ice from forming within the materials that made up Earth. Consequently, the solar wind would have driven all the water from this region before it could be incorporated into the planets. This new evidence meant astronomers needed to modify their model to explain why Earth has abundant liquid oceans. The simplest modification added a period of asteroid bombardment at least a hundred million years after Earth formed. A suitably large number of asteroids that originated outside the snowline could deliver enough water to account for the oceans and the water inside Earth. As with any good model, additional data must support the model's predictions. A review article in Nature provides data to support this updated model. Here are some of the results the paper presents: 1. 2. 3. The amount of zinc and potassium (compared to uranium) in terrestrial material and Martian meteorites falls well below the carbonaceous chondrites that represent the primordial material in the solar system. These elements condense at lower temperatures than uranium but at higher temperatures than water. Thus, if the material that formed Earth is depleted in zinc and potassium, it was certainly depleted of water also. The depletion of heavier isotopes of zinc matches the depletion of the lighter isotopes. If the volatile materials were lost during the accretion stage, the heavier isotopes would show less depletion compared to lighter isotopes because the Earth's gravity would bind them a little more tightly. Analysis of the radioisotopes hafnium and tungsten indicate that the impactor that formed the Moon occurred around 30 million years after Earth started forming. Additionally, rocks from the Moon exhibit 4. 5. less water than Earth's mantle. If Earth had a significant amount of water at the time the Moon formed, the moon would have ended with much more water. Analysis of xenon and lead isotopes indicate that most of these elements arrived at least 100 million years after the solar system started condensing. This is consistent with a model predicting a bombardment of asteroids that brings Earth its water. Without water, plate tectonics does not operate on a planet Earth's size. Without the addition of substantial water after the moon impact event, all of Earth's water would have been buried deep in Earth's interior (like the fate of Venus' initial water supply). However, a late verneer of water arriving from asteroid impacts would ensure that Earth's surface remains covered in water even after 4.5 billion years of plate tectonics. During that period, roughly half the water would be subducted into Earth's interior, matching measurements that indicate roughly an oceans' worth of water resides inside Earth. So what is the bottom line? Without an event that brought an abundance of asteroids from the outer regions of the solar system crashing to Earth, our planet would not have maintained a stable water cycle. Had this bombardment occurred too early, the water would have ended up buried deep inside the Earth instead of forming a life-essential liquid ocean. Only by the proper timing of this asteroid bombardment did Earth become habitable. Advances in our understanding of how our home developed continue to support the idea that a super Intellect worked to provide a place for humanity to reside. Subjects: Earth/Moon Design The Age(s) of the Continents August 6th, 2008 By Dr. Jeff Zweerink The Hebrew phrase “tōhū wābōhū” provides the first description of Earth given in Genesis 1. Many English Bible translations render this description as “formless and void (or empty)”. The Hebrew words imply that Earth’s surface was a desolate, undistinguishable ruin. Genesis 1:3 through Genesis 2:3 delineates how God transformed this wasteland into a variety of habitats teeming with life. One critical transformation involves breaking up the formless deep to form land upon which humans will live. On the third day of the creation week, Moses, the likely author of Genesis, declares that waters below the heavens were “gathered into one place” in order to “let the dry land appear”. In RTB’s creation model, this declaration means that scientists should find that the formation of a large, permanent continental landmass occurred within a definite time window (or burst) in Earth’s history. Additionally, that time window must close before the Cambrian explosion (which occurred around 540 million years ago), when complex multicellular life appeared on Earth. Past research on zircons revealed that most continental land dated to either 1.2, 1.9, 2.7, or 3.3 billion years ago. The clustering around these dates indicates that continental growth did occur in bursts. However, such clustering would also result from preferential preservation of crust that grew uniformly. More recent research adds further support to the idea that continental growth occurred in bursts. For continents to grow, regions of the mantle must melt and differentiate in order to provide the additional continental material. One particularly useful way to measure the melting of mantle material is the RheniumOsmium radioactive decay channel. A team of scientists using this decay channel discovered that mantle melting events also clustered around 1.2, 1.9, and 2.7 billion years ago. (No materials dating older than 3 billion years were used in the study.) The clustering of the continental ages and the mantle melting events around the same ages is extremely unlikely. Therefore, taken together, these results argue that the bulk of continent formation occurred in a time window between 3.3 and 1.2 billion years ago. Thus, these discoveries demonstrate a way the Creator could have “let the dry land appear” and add to the body of evidence supporting RTB’s creation model. Subjects: Earth/Moon Design Another Benefit for Life in Earthquakes December 7th, 2007 By Dr. David Rogstad Earthquakes are not particularly welcome by those who experience them (and Reasons To Believe would respond in a different manner in the event of a catastrophic event), nevertheless there are a number of very important benefits the planet derives from the processes that result in temblors. Earthquakes are a byproduct of plate tectonics, a theory in geology developed in recent years for explaining motions near the surface of the Earth. One of the benefits from plate tectonics is that Earth maintains the right levels of carbon dioxide (CO2) in the atmosphere to compensate for the Sun’s increasing luminosity. This is accomplished by what is called the carbonate-silicate cycle. CO2 is removed from the atmosphere through weathering. The weathered products are eventually drawn into the Earth’s interior via plate tectonics. Processes inside the Earth’s interior release the CO2 back into the atmosphere via volcanoes. While all aspects of this mechanism are not yet fully understood, it has been instrumental in providing a stable environment for life on the Earth for billions of years. New research provides yet another component that appears fine-tuned for life. In a letter in the September 27, 2007 issue of Nature together with a corresponding news release from the University of Bonn, Arno Rohrbach and his colleagues have discussed another mechanism similar to the carbonate-silicate cycle. It also depends on plate tectonics but, in this case, the mechanism controls the amount of oxygen on the surface of the Earth. Oxygen becomes bound up in various oxides which are then drawn into the Earth’s interior, where various processes result in its being incorporated into an exotic mineral called majorite. The results reported in this letter established that majorite functions as a kind of “reservoir” for oxygen, and when the majorite ascends nearer to the surface of the Earth it breaks down and releases its oxygen. Some of this oxygen also binds with hydrogen released from the interior of the Earth to form water. The authors have referred to the whole process as an “oxygen elevator.” They go on to say that “without the ‘oxygen elevator’ in its mantle the Earth would probably be a barren planet hostile to life. According to our findings, planets below a certain size hardly have any chance of forming a stable atmosphere with a high water content.” This research confirms the existence of one more finely tuned mechanism that depends on plate tectonics and contributes to an environment that can support life. It also gives humans one more reason to be appreciative rather than dismayed when we experience an earthquake that breaks some precious possessions beyond repair. Subjects: Earth/Moon Design A Planet’s Magnetic Field Protects Its Water January 30th, 2008 By Dr. Hugh Ross Disaster movies seem to be a staple for Hollywood. One such disaster movie that I found enjoyable (enough to watch it four times on my way to Japan) was The Core. The movie opens by showing some (unrealistic) consequences of Earth’s magnetic field disappearing (caused by a stoppage of the rotation of Earth’s core). The remainder of the movie details how a team of scientists attempt to restart Earth’s core and save the planet. While the film abounds with scientific inaccuracies and impossibilities, its premise highlights an important characteristic of Earth’s habitability, namely a strong magnetic field. Venus and Earth are remarkably similar in composition and size. Venus has 81% of Earth’s mass, and its radius and density are only about 5% smaller than Earth’s. Due to their forming in a similar section of the solar system, both Earth and Venus likely started covered with water and had essentially identical atmospheres. While these two “sister planets” began similarly, they could not be more different today in terms of their habitability. Earth’s atmosphere consists of mainly nitrogen (78%) and oxygen (21%), with trace amounts of other gases like carbon dioxide and helium. Additionally, abundant clouds of water vapor fill the skies. In stark contrast, Venus’ atmosphere is comprised of mostly carbon dioxide (96%) and nitrogen (3%) with dense clouds of sulfuric acid! Earth’s atmosphere causes surface temperatures around 70oF, Venus’ surface sits around 800oF. What caused this difference? Many factors contribute to the disparity between Earth and Venus, but recent results from the Venus Express satellite highlight one of the more important differences. Because Earth rotates once every 24 hours, this motion causes its iron core to generate a strong magnetic field. This magnetic field shields Earth from cosmic rays, in addition to protecting Earth’s atmosphere from the solar wind. Venus rotates only once for every 243 Earth days. Consequently, Venus has no significant magnetic field to shield its atmosphere from the solar wind. Without a magnetic shield, the solar wind strips away all the water from Venus’ surface. Ultraviolet radiation from the Sun breaks water molecules down into two hydrogen ions and one oxygen ion (atoms with an electric charge due to an extra electron or a deficiency of an electron). The charged particles in the solar wind accelerate these ions and strip them from the atmosphere. However, Earth’s magnetic field deflects the solar wind around the atmosphere so the ions are not stripped off into space. The results published in Nature from the Venus Express mission demonstrate that the ions coming from Venus match the composition expected if water is being stripped from its atmosphere. The water currently being stripped from Venus arises from recent small comet impacts that deposit water in Venus’ atmosphere (similar processes occur on Earth also). However, the solar wind has also stripped away all the water that Venus started with over four billion years ago. These findings highlight how a habitable planet must not only be similar in size and composition to Earth, but it also must have a strong magnetic field. Thus, one must read optimistic announcements such as this with a bit of caution. Subjects: Earth/Moon Design Earth’s Primordial Atmosphere Must Be Fine-Tuned March 14th, 2011 By Dr. Hugh Ross An adult human can last 40 days without food, a week without any sleep, three days without water, but only five minutes without air. Yet nothing is more taken for granted than the air we breathe. However, not just any air will do—it must be exquisitely designed to meet our needs. Too little oxygen in the atmosphere will kill us, as will too much. The same kind of fine-tuning needs apply to atmospheric nitrogen, carbon dioxide, ozone, and water vapor. A team of eight planetary scientists recently discovered that Earth did not always possess its present-day atmospheric composition.1 (Table 1 lists the different chemicals and their relative abundances for the presentday atmosphere.) The team also found that differing atmospheric compositions throughout Earth’s history played crucial roles in enabling our planet to support life for several billion years and in particular to make advanced life possible. Table 1: The Composition of Earth’s Present-Day Atmosphere Gas Component Percent Abundance by Volume nitrogen 77.77 oxygen 20.86 argon 0.93 water vapor 0.40 (1–4% at surface) carbon dioxide 0.039 neon 0.002 (all other gas components combined measure less than 0.001) To determine the impact of the Sun’s radiation on Earth’s atmosphere during the Sun’s first 800 million years, the planetary science team took advantage of astronomers’ detailed knowledge of the Sun’s properties during that epoch (see figure 1). They showed that if Earth’s atmosphere were the same then as it is now, the solar wind and ultraviolet radiation would have completely removed the planet’s atmosphere within a few million years or less. Since this removal did not happen, the team concluded that Earth’s primordial atmosphere must have differed substantially from its present one. Figure 1: Level of Solar Flaring throughout the Sun’s Burning History The intensities of the Sun’s ultraviolet radiation, flaring activity, and wind started off at very high levels, subsided to minima when the Sun reached 4.6 billion years old (its present age), and then will gradually increase hereafter. Image credit for background image: NASA The team then produced calculations demonstrating that the only reasonable scenario for explaining why the Sun’s radiation did not remove Earth’s primordial atmosphere was that the early Earth’s atmosphere was at least a hundred times richer in carbon dioxide. Such an extremely carbon-dioxide rich atmosphere, the team proved, would have confined our planet’s upper atmosphere to within Earth’s magnetosphere. (The magnetosphere shields Earth’s atmosphere from destructive damage by solar radiation.) The extra carbon dioxide in the primordial atmosphere serves another benefit for Earth’s life. It trapped much more of the Sun’s heat. Since the Sun was about 15 percent dimmer at the time (see figure 2), such enhanced heat-trapping capacity enabled life to exist at that time. With the Sun getting progressively brighter throughout the past 3.5 billion years, it then became crucial for life’s ongoing existence that Earth’s atmosphere lose carbon dioxide progressively. Such losses posed no problem for the preservation of the planetary atmosphere because as the Sun brightened, its radiation also became progressively less destructive. Figure 2: The Sun’s Brightness throughout Its Burning History The luminosity or brightness levels are all relative to the Sun’s present luminosity (dotted line). Image credit for background image: Hugh Ross Though not mentioned in their paper, the planetary science team’s research adds to the already overwhelming evidence for the rare Earth doctrine. Not only must the various constituents that make up Earth’s present-day atmosphere be carefully fine-tuned, those constituents must vary in highly specified ways throughout all of Earth’s history. The team’s research provides yet one more example of how the more scientists learn about Earth’s properties the more evidence they establish that our home planet was supernaturally designed for life and especially for human beings and their global, high-technology civilization. Endnotes: 1. H. I. M. Lichtenegger et al., “Aeronautical Evidence for Higher CO2 Levels during Earth’s Hadean Epoch,” Icarus 210 (November 2010): 1–7. Subjects: Earth/Moon Design, Extrasolar Planets Elemental Evidence of Earth’s Divine Design March 1st, 2010 By Dr. Hugh Ross The familiar adage “You can’t have too much of a good thing” doesn’t hold true for planet Earth. Too much water, for example, or too much carbon would destroy Earth’s ability to support advanced life. On the other hand, too little of certain “bad” things, elements generally considered poisonous to life, would also ruin Earth’s chances to serve as a life site. Many of these elements must be present on Earth in “just right” quantities or we wouldn’t be here. When Carl Sagan and others first claimed that among the billions and billions of stars in the universe we’d be sure to find millions (or more) of life sites, they gave the impression that our home is just an ordinary, notreally-unusual planet. The past few decades of research, however, have shown us just the opposite: in its array and abundances of various elements and compounds, Earth is, in fact, extraordinary. Given its size and distance from the Sun, Earth holds an unusual and unexpected quantity of virtually every element and compound researchers can measure and compare—and especially in the case of elements and compounds vital to the existence of advanced life. Figure 1: Cosmic Abundance history of Uranium and Thorium Supernovae deliver uranium and thorium to the interstellar medium. However, the supernovae eruption rate declines as the universe ages. Eventually the delivery rate fails to keep pace with radiometric decay. For Earth to receive a maximal amount of uranium and t horium it must form when the cosmic abundance of those elements reaches a peak. (Credit for background image of the galaxy NGC 6217: NASA/ESA/Hubble SM4 ERO Team) Astronomers have begun to gain some understanding as to how Earth came to be so different. It starts with the timing of the solar system’s origin. For example, as I often mention in my talks, our solar system formed at that moment in cosmic history when uranium and thorium reached their peak abundance (see Figure 1). Since that time, their production through supernova eruptions (the only cosmic source of uranium and thorium) has failed to keep pace with their radioactive decay. So their availability for incorporation into newly forming planets has decreased. The significance of this timing is that uranium and thorium are essential elements for driving Earth’s plate tectonics. Thanks to the timing of Earth’s formation and a number of subsequent events in the Sun’s and Earth’s early history (which will be described below), Earth was supplied with enough uranium and thorium to sustain strong, and enduring plate tectonics, which in turn provide for a just-right balance of oceans and continents—critical surface features for the recycling of nutrients and the existence of advanced life (see Figure 2). Figure 2: Growth of Continents on Earth's Surface: The Vertical axis shows the percentage of Earth's surface area comprised of continents. As the graph indicates, landmasses grew rapidly around the 2-billion-year mark and have increased gradually since then. The Current coverage of Earth's surface by continents is ideal for the efficient recycling of nutrients and for the support of global human civilization. (Credit for background image of Earth: NASA) The solar system formed not only with the best possible timing but also, simultaneously, in the best possible location for the sake of humanity. Both time and place influence the relative quantities of particular metal isotopes in early Earth’s composition. As British planetary astronomers Jamie Gilmour and Ceri Middleton noted, the exceptionally high ratio of aluminum-26 to aluminum-27 (4.5-5 x 105) at the solar system’s origin site is “difficult to produce in models of star formation.”1 In other words, it’s highly unusual and unexpected. Four astronomers at the Universities of Hawaii and Colorado determined how the primordial solar system became so exceptionally enriched with aluminum-26.2 A prior generation of giant stars formed near enough to the giant molecular cloud from which the Sun and planets later emerged to shower it with aluminum-26 and other important isotopes (via “Wolf-Rayet” winds). If either the timing or location of the solar system’s formation were adjusted ever so slightly sooner or later or nearer or farther relative to these massive stars, the forming solar system would have been destroyed (or severely damaged) or would have been inadequately enriched with aluminum-26. And yet without this high ratio of aluminum-26 to aluminum-27, there would have been no “thermal processing of planetesimals,”3 to use the words of Gilmour and Middleton. This would mean no heat pulse to drive off the dangerously high (for advanced life) quantity of volatile gases—carbon dioxide, carbon monoxide, nitrogen oxides, water, etc. from the primordial solar system. As the researchers pointed out, without this heat pulse Earth would have retained far too much water for continents to be possible and far too much carbon for an atmosphere to be breathable. Meanwhile, to acquire sufficient quantities of other elements essential for advanced life, the emerging solar system had to form at the just right location and time relative to two different types of supernovae as well as to several objects called “asymptotic giant branch stars.”4 All the requirements described thus far mandate that the Sun and its planets begin to form in a large and dense star cluster. However, once it’s appropriately enriched with the uranium, thorium, aluminum-26, and other essential elements, the solar system must be ejected from this cluster because advanced life cannot tolerate close encounters with other stars.5 Earth, itself, demands special circumstances during its formative years. One of the most stunning was an event described in 2004 by Robin Canup. Her theory stated that a planet slightly more massive than Mars (Mars = 0.107 Earth masses) collided with the newly formed Earth—at an impact angle of about 45 degrees and impact velocity of less than 4 kilometers/second.6 This low-velocity collision brought about four significant-foradvanced-life changes: (1) it blasted away most of Earth’s water and atmosphere; (2) it ejected light element material and delivered heavy elements; (3) it transformed both the interior and exterior structure of Earth; and (4) it led to the formation of Earth’s exceptionally large Moon. 7 (Canup improved her model in 2008, concluding that a somewhat larger collider impacted a retrograde rotating proto-Earth, while further confirming her original findings.)8 Approximately 650 million years after that collision, yet another pivotal (for advanced life) event occurred. An orbital resonance between Jupiter and Saturn disrupted the asteroids and comets of the Kuiper Belt, triggering an episode called the Late Heavy Bombardment9 (see Figure 3). Over a period of less than a hundred million years, some 17,000 impactors smashed into Earth, together depositing 200 tons of material on every square yard of Earth’s surface.10 Many of these impactors pierced through Earth’s crust. In the final analysis, this intense period of bombardment, as hellish as it may seem, brought about a chemical transformation, both interior and exterior, that favored the elemental needs of later advanced life. Figure 3: The Late Heavy Bombardment Thousands of asteroids and comets pummeled Earth within a relatively brief time period some 3.9 billion years ago. This bombardment chemically transformed both Earth’s interior and exterior. (Credit for images of asteroids and comets: NASA; credit for the deep space background: NASA/ESO; remaining artwork is the author’s.) For a more complete list of Earth’s extraordinary quantities of chemical elements and compounds, see the table below. As you read it, be aware that every one of Earth’s exceptional abundances discovered to date has proved crucial for the support of life—advanced life, in particular. The evidence for the supernatural, superintelligent, super-intentional design of Earth continues to mount. Figure 4: Earth’s “Unobtainium,” Uraninite Uraninite, also known as pitchblende, is the most uranium-rich ore on Earth. Uranium is more abundant on Earth than tin, antimony, or cadmium. Its exceptionally high abundance makes possible nuclear fission power plants. One kilogram of uranium-235 can yield up to 80 trillion joules of energy. And, because of its density, hardness, ease of use in machining and casting, and low cost, depleted uranium finds a wide range of industrial applications. [Photo Credit: Joachimsthal, Bohemia, Czechoslovakia Creative Commons (http://minerals.no-ip.com/Minerals/html/Pichblende1.html)] Table: Earth’s Anomalous Abundances The twenty-five elements listed below must exist on Earth in specific abundances for advanced life and/or support of civilization to be possible. For each listed element the number indicates how much more or less abundant it is, by mass, in Earth’s crust, relative to magnesium’s abundance, as compared to its average abundance in the rest of the Milky Way Galaxy, also relative to the element magnesium. Asterisks denote “vital poisons,” essential elements that if too abundant would be toxic to advanced life, but if too scarce would fail to provide the quantities of nutrients essential for advanced life. The water measure compares the amount of water in and on Earth relative to the minimum amount the best planet formation models would predict for a planet the mass of Earth orbiting a star identical to the Sun at the same distance from the Sun. 11 carbon* 1,200 times less cobalt* 5 times less nitrogen* 2,400 times less selenium* 30 times less fluorine* 50 times more yttrium 50 times more sodium* 20 times more zirconium 130 times more aluminum 40 times more niobium 170 times more phosphorus* 4 times more12 moybdenum* 5 times more sulfur* 60 times less13 tin* 3 times more potassium* 90 times more iodine* 3 times more calcium 20 times more gold 5 times less titanium 65 times more lead 170 times more vanadium* 9 times more uranium 16,000 times more chromium* 5 times less thorium 23,000 times more nickel* 20 times less water 250 times less __________ Subjects: Earth/Moon Design, Extrasolar Planets Questions about this topic? Big Collision, Beautiful Moon November 1st, 2006 By Administrator 11/1/2006 by Dr. Jeff Zweerink A demolition expert surveys the building designated for destruction. With one swing of the wrecking ball, he must bring down the building without scattering the debris off the property. Such a precise operation requires the right size wrecking ball hitting at just the right speed. Hitting too high only removes the roof; too low and the ground absorbs all the wrecking force. The possibilities for a failed demolition far exceed the ways to succeed. After exacting calculations, the wrecking ball scores a direct hit, transforming the building into an easily cleaned-up pile of debris. About 50 million years after the formation of the solar system, a similarly fine-tuned collision between Earth and a Mars-sized body occurred. However, instead of destroying Earth, the collision provided raw materials for the formation of Earth's moon. The collision ejected debris into orbit that eventually coalesced into the Moon. Recent high-resolution simulations of the impact event1 confirm the fine-tuning of the impact to insure the survival of Earth, formation of the Moon, and transformation of Earth's atmosphere.2 The simulations show that the debris ejected from Earth must have consisted primarily of solid or liquid material-not gas-or else the debris disk would have dissipated too quickly to coalesce into a Moon-sized satellite. A larger impactor would have generated more energy during the collision and, consequently, more vaporized, gaseous material in the debris disk. However, a smaller impactor would not enrich Earth with the necessary heavy elements to drive long-standing plate tectonics nor provide sufficient energy to completely eject Earth's life-suffocating primordial atmosphere into space. (This gas does not become part of the debris disk, but is completely removed from the Earth-Moon system.) Thus, if the impactor were larger or smaller, the capacity of Earth to support advanced complex life (like humans) or abundant, long-standing microbial life rapidly diminishes. Additionally, the authors note that if a planet is too large, it cannot have a moon formed by a giant impact event. The Moon-forming impact requires a just-right-sized impactor striking Earth at the justright speed, at the just-right location, with the just-right angle, and at the just-right time. Just as the demolition expert must carefully prepare his work in order to avoid failure, so the Moon-forming impact required a number of just-right factors in order to succeed. As scientific advances continue to reveal more fine-tuning factors, the idea that the impact happened purely by chance seems less and less feasible. On the other hand, such fine-tuning comports well with RTB's biblical creation model, in which a supernatural Creator intervenes to ensure Earth's long-standing habitability in preparation for humankind. References 1. 2. Keiichi Wada, Eiichiro Kokubo, and Junichiro Makino, "High-Resolution Simulations of a Moon-Forming Impact and Postimpact Evolution," Astrophysical Journal 638 (2006): 1180-86. Kevin Zahnle, "Being There," Nature 433 (2005): 814-15; Hidenori Genda and Yutaka Abe, "Enhanced Atmospheric Loss on Protoplanets at the Giant Impact Phase in the Presence of Oceans," Nature 433 (2005): 842-44. Subjects: Earth/Moon Design Planet Formation: Problems with Water, Carbon, and Air January 12th, 2009 By Dr. Hugh Ross Thanks to a study from two MIT planetary scientists, the rare planet doctrine now finds additional support. This is the conclusion that Earth has many unique, apparently designed features that enable it to support life and, in particular, advanced life. The reseachers model degassing during the accretion phase of planetary formation for planets ranging in mass from 1 to 30 times the mass of Earth.1 Their study was motivated in part by the recent discovery of several “super-Earths,” planets outside the solar system ranging in mass from 3 to 10 times Earth’s mass. These scientists begin by pointing out that planets in general possess three different opportunities for gaining an atmosphere: capture from the protoplanetary disk surrounding their primordial star, degassing during the planetary accretion process, or later degassing resulting from the planet’s tectonic activity. While capture from the protoplanetary disk certainly is the dominant means for the buildup of atmospheres around the gas giant planets, planetary scientists are still uncertain of the degree to which such capture plays a role for planets the size of Earth or a few times larger. Thus, the MIT team decided to consider only the role of degassing during the planetary accretion process. They based their models on measurements of the bulk compositions in the most primitive meteorites found in the solar system. These ancient remnants of the solar system’s protoplanetary disk represent the material from which Earth formed. They contain up to 20 percent of water by mass. The team used the range of water and carbon found in such meteorites and modeled how much of it would be retained in the formation process by Earths and super-Earths. The scientists determined that degassing during accretion alone would result in water and carbon compounds making up to 20 percent and 5 percent of the mass of Earths and super-Earths, respectively. They found, too, that using even modest estimates of water and carbon in the meteorites resulted in Earths and super-Earths ending up with very deep oceans and very thick atmospheres. Both results pose major problems for potential habitability. Due to deep oceans, no conceivable amount of plate tectonic activity would ever produce continents. Without continents there would be no possibility for land life. Additionally, many important nutrient-recycling mechanisms would be absent. Thick atmospheres loaded with carbon compounds would trap tremendous amounts of heat, and would result in atmospheric pressures that would make lungs inoperable and block out so much stellar light as to impede photosynthesis. This study underscores just how anomalous our Earth is. For a planet as large as it is and as far away from its star, Earth is miraculously water- and carbon-poor. Water makes up just 0.02 percent of Earth’s mass; carbon just 0.003 percent. While water and carbon are essential for life, too little or too much proves deadly, especially in the case of advanced life. Earth possesses the just-right amount of each. Furthermore, the report demonstrates that Earth, like all planets its size and distance from its star, started off with a huge amount of water and carbon. Thanks to an exquisitely designed collision event early in the planet’s history, Earth lost just the right amounts of water and carbon. This event also led to the formation of the Moon.2 The MIT team’s research study illustrates a Christian apologetics principle. It shows that the more we learn about the physics of extrasolar planetary systems, the more evidence we accumulate for the supernatural, super-intelligent design of the Milky Way Galaxy, the solar system, and Earth for the benefit of all life on Earth, both simple and complex.