jace14095-sup-0001-SupInfo
advertisement

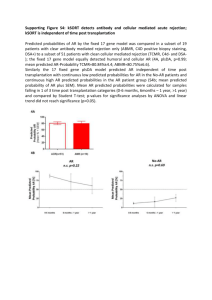
Supplementary Materials LIST OF TABLES Table S1. The true and retrieved input parameters from numerically generated temperature profiles predicted from the combined conduction and radiation heat transfer model for different data sets of a, b, 1 , 2 , and Tf,R. -1- LIST OF FIGURES Figure S1. (a) Spectral absorption coefficient of gray and low-iron glass reported in the literature [23] as well as the corresponding band coefficients used to predict steady-state temperature profile in the glassmelts; (b) the predicted steady-state temperature profiles in the gray and low-iron glassmelts slab for various cut-off wavelength of cut = 5 m and 8 m and for furnace temperature Tf = 1400oC. Figure S2. Estimated spectral normal emissivity of the crucible wall based on the optical properties of various soda-lime silicate glass reported in the literature [23] and the optical properties of alumina (the crucible was made of ~97% alumina) reported in the literature [30]. Figure S3. Spectral absorption coefficient of (a) float/clear glass and (b) green glass reported in Ref. [23] as well as the corresponding eight bands approximation of absorption coefficient used in the present study. Figure S4. Comparison between the predicted steady-state temperature profiles and the results reported by Lee and Viskanta [24], (a) float/clear glass, and (b) green glass. Figure S5. Spectral absorption coefficient of gray and low-iron glass reported in Ref. [23] and the corresponding bandwise Rosseland mean two-band absorption coefficient ( R,1 , R ,2 ) defined by Equation (12). Figure S6. Comparison of the temperature profiles predicted by using either the spectral absorption coefficient [23] or the two-band Rosseland mean absorption coefficient approximation for (a) gray and (b) low-iron soda-lime silicate glass for Tf = 1400oC. -2- Figure S7. Comparison between experimental data and numerical temperature profiles predicted using the retrieved properties reported in Table 1 for gray soda-lime silicate glassmelt (composition 1) at furnace temperature Tf of (a) 1350oC, (b) 1400oC, (c) 1450oC and (d) 1500oC. Figure S8. Comparison between experimental data and numerical temperature profiles predicted using the retrieved properties reported in Table 2 for green soda-lime silicate glassmelt (composition 2) at furnace temperature Tf of (a) 1300oC, (b) 1350oC, (c) 1400oC, (d) 1450oC, and (e) 1500oC. Figure S9. Comparison between experimental data and numerical temperature profiles predicted using the retrieved properties reported in Table 3 for clear soda-lime silicate glassmelt (composition 3) at furnace temperature Tf of (a) 1300oC, (b) 1350oC, (c) 1400oC, (d) 1450oC, and (e) 1500oC. Figure S10. Comparison between experimental data and numerical temperature profiles predicted using the retrieved properties reported in Table 4 for low-iron soda-lime silicate glassmelt (composition 4) at furnace temperature Tf of (a) 1350oC, (b) 1400oC, and (c) 1450oC. -3- Table S1. The true and retrieved input parameters from numerically generated temperature profiles predicted from the combined conduction and radiation heat transfer model for different data sets of a, b, 1 , 2 , and Tf,R. Parameter Tf,R (oC) Case 1 Exact Retrieved a (W/m∙K) 1 (m-1) 2 (m-1) Case 3 Case 4 1400 1400 1.20 1.70 1400 1400 1400 Exact Retrieved b (W/m∙K2) Case 2 1.14 1.35 1.35 6.24×10-4 Exact Retrieved 4.39×10-4 5.20×10-4 7.20×10-4 2.70×10-4 Exact 20 40 150 250 Retrieved 18.8 40.2 150.3 251.3 413.5 442.7 Exact Retrieved 450 499.1 -4- 407.5 Absorption coefficient,m (a) 106 10 5 10 4 10 3 10 2 10 1 10 0 10 Gray, spectral Gray, band Low-iron, spectral Low-iron, band -1 0 1 2 3 4 5 6 7 8 wavelength, (m) (b) 1395 Low-iron, cut = 5 m Low-iron, cut = 8 m Gray, cut = 5 m Gray, cut = 8 m o Temperature, T( C) 1390 1385 1380 o Tf = 1400 C 1375 0.0 0.4 0.8 1.2 1.6 2.0 Location, x (cm) Figure S1. (a) Spectral absorption coefficient of gray and low-iron glass reported in the literature [23] as well as the corresponding band coefficients used to predict steady-state temperature profile in the glassmelts; (b) the predicted steady-state temperature profiles in the gray and lowiron glassmelts slab for cut-off wavelength of cut = 5 m and 8 m and for furnace temperature Tf = 1400oC. -5- The effect of cut-off wavelength cut was investigated by comparing the predicted steadystate temperature profile in a plane-parallel slab of thickness of 2 cm for cut equal to either 5 m or 8 m . Two types of glassmelt were investigated namely gray and low-iron glassmelts (the extreme cases). The spectral absorption coefficient of these glasses were taken from the literature [23], Figure S9(a) shows the corresponding band absorption coefficients used to predict the temperature profile. The true thermal conductivity of both glasses was taken as kc(T) = 1.14 + 6.24×10-4T [22], the furnace temperature Tf was set as 1400oC. The total heat flux across the gray and low-iron glassmelts was taken arbitrarily as 15,000 W/m2 and 12,000 W/m2, respectively. Figure S9(b) shows the predicted steady-state temperature profile in the glassmelts. For both gray and low-iron glassmelts, the maximum difference in the temperature profiles predicted for cut-off wavelength cut = 5 m and 8 m was less than 1oC. These results indicate that choosing cut = 5 m , as performed in the literature [22, 24-25] gives acceptable temperature profile predictions. -6- Spectral normal emissivity, n, 1.000 lowiron clear green gray 0.998 0.996 0.994 0.992 0.990 0 1 2 3 Wavelength, (m) 4 5 Figure S2. Estimated spectral normal emissivity of the crucible wall based on the optical properties of various soda-lime silicate glass reported in the literature [23] and the optical properties of alumina (the crucible was made of ~97% alumina) reported in the literature [30]. -7- 10 4 Spectral absorption coefficient [23] Band model approximation -1 Absorption coefficient,(m ) (a) 10 3 10 2 10 1 Float(clear) glass 0 10 4 10 3 10 2 2 3 4 Wavelength, (m) 5 Spectral absorption coefficient [23] Band model approximation -1 Absorption coefficient,(m ) (b) 1 Green glass 10 1 0 1 2 3 4 Wavelength, (m) 5 Figure S3. Spectral absorption coefficient of (a) float/clear glass and (b) green glass reported in Ref. [23] as well as the corresponding eight bands approximation of absorption coefficient used in the present study. -8- (a) 1800 Temperature, T (K) 1600 1400 1200 Float/clear glass H =0.01 m, Lee et al. [24] H =0.01 m, Present study H =0.1 m, Lee et al. [24] H =0.1 m, Present study H =1.0 m, Lee et al. [24] H =1.0 m, Present study 1000 800 600 400 0.0 0.2 0.4 0.6 Location, x/L 0.8 1.0 (b) 1800 Temperature, T (K) 1600 1400 1200 Green glass H =0.01 m, Lee et al. [24] H =0.01 m, Present study H =0.1 m, Lee et al. [24] H =0.1 m, Present study H =1.0 m, Lee et al. [24] H =1.0 m, Present study 1000 800 600 400 0.0 0.2 0.4 0.6 Location, x/L 0.8 1.0 Figure S4. Comparison between the predicted steady-state temperature profiles and the results reported by Lee and Viskanta [24], (a) float/clear glass, and (b) green glass. -9- 4 10 3 10 2 -1 Absorption coefficient, (m ) 10 2.8 m gray, 10 gray, R 1 low-iron, 10 low-iron, R 0 0 1 2 3 4 5 wavelength, (m) Figure S5. Spectral absorption coefficient of gray and low-iron glass reported in Ref. [23] and the corresponding bandwise Rosseland mean two-band absorption coefficient ( R,1 , R ,2 ) defined by Equation (12). - 10 - (a) 1400 Spectral calculation Two bands (R1, R2) o Temperature, T( C) 1380 1360 1340 1320 1300 0 (b) 2 o 6 8 10 Location, x (cm) 12 14 1400 Spectral calculation Two bands (R1,R2) 1395 Temperature, T ( C) 4 1390 1385 1380 1375 1370 1365 1360 0 2 4 6 8 10 12 Location, x (cm) 14 16 Figure S6. Comparison of the temperature profiles predicted by using either the spectral absorption coefficient [23] or the two-band Rosseland mean absorption coefficient approximation for (a) gray and (b) low-iron soda-lime silicate glass for Tf = 1400oC. - 11 - (a) 1360 (b) 1400 Experimetal data Predicted data 1340 1320 Predicted data 1360 Temperature, T( C) 1300 o o Temperature, T( C) Experimetal data 1380 1280 1260 1240 1220 o Tf = 1350 C 1200 1340 1320 1300 1280 o Tf = 1400 C 1260 1180 1240 0 2 4 6 8 10 12 14 0 2 4 Location, x (cm) 10 12 14 (d) 1520 Experimetal data Predicted data 1440 Experimetal data 1500 Predicted data 1480 o Temperature, T( C) 1420 o 8 Location, x (cm) (c) 1460 Temperature, T( C) 6 1400 1380 1360 1340 o Tf = 1450 C 1320 1460 1440 1420 1400 o Tf = 1500 C 1380 1300 1360 0 2 4 6 8 10 12 14 Location, x (cm) 0 2 4 6 8 10 12 14 Location, x (cm) Figure S7. Comparison between experimental data and numerical temperature profiles predicted using the retrieved properties reported in Table 1 for gray soda-lime silicate glassmelt (composition 1) at furnace temperature Tf of (a) 1350oC, (b) 1400oC, (c) 1450oC and (d) 1500oC. - 12 - (a) 1280 (b) 1340 Experimetal data Predicted data 1260 o Temperature, T( C) o Predicted data 1300 1240 Temperature, T( C) Experimetal data 1320 1220 1200 1180 1160 o Tf = 1300 C 1140 1280 1260 1240 1220 1200 o Tf = 1350 C 1180 1120 1160 0 2 4 6 8 10 12 14 16 0 2 4 Location, x (cm) 10 12 14 16 (d) 1440 Experimetal data Predicted data 1360 Experimetal data 1420 Predicted data 1400 o Temperature, T( C) 1340 o 8 Location, x (cm) (c) 1380 Temperature, T( C) 6 1320 1300 1280 1260 o Tf = 1400 C 1240 1380 1360 1340 1320 o Tf = 1450 C 1300 1220 1280 0 2 4 6 8 10 12 14 16 Location, x (cm) 0 2 4 6 8 10 12 14 16 Location, x (cm) Figure S8. Comparison between experimental data and numerical temperature profiles predicted using the retrieved properties reported in Table 2 for green soda-lime silicate glassmelt (composition 2) at furnace temperature Tf of (a) 1300oC, (b) 1350oC, (c) 1400oC, (d) 1450oC, and (e) 1500oC. - 13 - (e) 1480 Experimetal data Predicted data 1440 o Temperature, T( C) 1460 1420 1400 1380 o Tf = 1500 C 1360 1340 0 2 4 6 8 10 12 Location, x (cm) Figure S8. (Continued) - 14 - 14 16 (a) 1260 (b) 1320 Experimental data Predicted data Experimental data Predicted data 1310 Temperature, T( C) 1240 1300 o o Temperature, T( C) 1250 1230 1220 1210 o Tf = 1300 C 1290 1280 1270 o Tf = 1350 C 1260 1200 1250 0 2 4 6 8 10 12 14 16 0 2 4 Location, x (cm) 8 10 12 14 16 Location, x (cm) (c) 1380 (d) 1430 Experimental data Predicted data 1370 Experimental data Predicted data 1420 1410 o Temperature, T( C) 1360 o Temperature, T( C) 6 1350 1340 1330 o Tf = 1400 C 1320 1310 1400 1390 1380 o Tf = 1450 C 1370 1360 0 2 4 6 8 10 12 14 16 Location, x (cm) 0 2 4 6 8 10 12 14 16 Location, x (cm) Figure S9. Comparison between experimental data and numerical temperature profiles predicted using the retrieved properties reported in Table 3 for clear soda-lime silicate glassmelt (composition 3) at furnace temperature Tf of (a) 1300oC, (b) 1350oC, (c) 1400oC, (d) 1450oC, and (e) 1500oC. - 15 - (e) 1490 Experimental data Predicted data 1470 o Temperature, T( C) 1480 1460 1450 1440 o Tf = 1500 C 1430 1420 0 2 4 6 8 10 12 Location, x (cm) Figure S9. (Continued) - 16 - 14 16 (b) 1390 (a) 1340 Experimental data Predicted data Experimental data Predicted data 1380 Temperature, T( C) 1320 o o Temperature, T( C) 1330 1310 1300 o Tf = 1350 C 1290 1280 1370 1360 1350 o 1340 Tf = 1400 C 1330 0 2 4 6 8 10 12 14 16 0 2 4 Location, x (cm) 6 8 10 12 14 16 Location, x (cm) (c) 1440 Experimental data Predicted data o Temperature, T( C) 1430 1420 1410 1400 o Tf = 1450 C 1390 0 2 4 6 8 10 12 14 16 Location, x (cm) Figure S10. Comparison between experimental data and numerical temperature profiles predicted using the retrieved properties reported in Table 4 for low-iron soda-lime silicate glassmelt (composition 4) at furnace temperature Tf of (a) 1350oC, (b) 1400oC, and (c) 1450oC. - 17 - Estimate of the uncertainties for the retrieved properties shown in Figure (9b): Figure 9(b) shows the average values of 1,av , 2,av , and the associated uncertainties 1 , 2 for the four types of soda-lime silicate glassmelts. The uncertainties 1 and 2 were estimated as 1 NF i i , j i,av NF 1 j 1 1/2 2 , with i= 1 or 2 where NF is the number of datasets collected at different furnace temperatures and i , j is the associated band absorption coefficient retrieved. Here, NF = 6 for furnace temperature Tf ranging from 1300oC to 1550oC by 50 oC increments. - 18 -