3.03 The Mole Introduced HO
advertisement

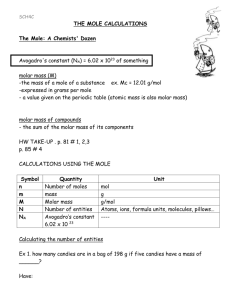
SCH 4C The Mole Introduced We frequently refer to common measures casually... Couple means ______ Few means ______ Dozen means ______ Baker’s Dozen means ______ Ream means ______ LORD means ______ 1 Mole means ________________________________ The symbol for mole is: ______ and the unit for mole is ______ A mole can be anything….eggs, particles, molecules, atoms, formula units, etc. In chemistry, 1 mole refers to specific particles. The name of the particle depends on the identity of the substance. Molecules – _____________________ bonded compounds. Usually a _____________________ and a _____________________. The _____________________ of water is the particle that is counted. Therefore 1 mole of water has 6.02 X 1023 _____________________. Formula Units - _____________________ bonded compounds. Usually a _____________________ and a _____________________. The _____________________ of salt is the particle that is counted. Therefore 1 mole of water has 6.02 X 1023 _____________________. Atoms - _____________________. Found on the _____________________ but are not part of _____________________ (the diatomic gases). The _____________________ of copper is the particle that is counted. Therefore 1 mole of copper has 6.02 X 1023 _____________________. Pop Quiz. Identify the unit for each substance. Methane (CH4) Magnesium chloride Aluminum Butane (C4H10) Hydrogen peroxide Sodium nitrate Calcium Mole to Particle Calculations A calculation line is useful when dealing with moles. MOLES Example 1: There are 0.50 mol of methane, CH4 in a flask. How many molecules are present? Example 2: How many molecules are found in 1.25 mol of CO2? Example 3: How many moles are found in 5.83 x 1024 molecules of CO2? Example 4: How many moles are found in 3.57 x 1025 atoms of zinc? Practice: 1. Determine the number of representative particles in each of the following. Show your work. A. 0.250 mol silver B. 8.56 x 10-3 mol NaCl C. 35.4 mol CO2 D. 0.425 mol N2 2 . Determine the number of moles in each of the following. Show your work. A. 3.25 x 1020 atoms Pb B. 4.96 x 1024 molecules glucose C. 1.56 x 1023 formula units NaOH D. 1.25 x 1025 Cu2+ ions 3. Make the following conversions. Show your work. A. 1.51 x 1015 atoms Si to mol Si B. 4.25 x 10-2 mol NO2 to molecules NO2 C. 8.95 x 1025 molecules CCl4 to mol CCl4 D. 5.90 mol Ca to atoms Ca