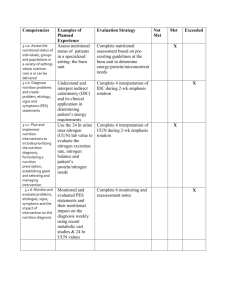
File - Andria M. Keating`s Professional Dietetics Portfolio
advertisement

UNIVERSITY OF VIRGINIA HEALTH SYSTEM DIETETIC INTERNSHIP Optimum Nutritional Management of Hospitalized Pediatric Oncology Patients: A Case Series Based QI Research Project Andria M. Keating, RD Intern Capstone Project Preceptor: Allie E. Hubbard, RD May 2013 Table of Contents I. Abstract…………………………………………………….. II. Introduction………………………………………………… 4-5 III. Literature Review…………………………………………... 6 - 34 IV. Purpose of study…………………………………………… 35 V. Mixed Methods Case Series Study Methodology…………. 36 - 39 VI. Results…………………………………………………….... 40 - 74 VII. Discussion…………………………………………………... 75 - 79 VIII. Conclusion and Recommendations…………………………. 80 - 81 IX. References…………………………………………………… 82 - 85 X. Appendix A: Nutritional Assessment…………………………. 86 - 87 XI. Appendix B- Pediatric Oncology Nutrition Data Form………. 88 XII. Appendix C- UVA Health System Interview Guide………...... 89 - 94 XIII. Appendix D: Algorithm for Nutritional Intervention…………. 95 1 2-3 ABSTRACT Introduction - As medical advancements in the field of pediatric oncology have developed, the overall survival rate for this population has improved and focus has shifted to the increased importance of supportive care treatments for children with cancer. Unfortunately, despite medical advancements, pediatric oncology patients remain susceptible to malnutrition due to risk of rapid weight loss, which can lead to a poor prognosis. The implementation of timely and adequate nutritional support, when indicated, is important in promoting optimal nutrition status and quality of life for patients. Nutritional support is often given to the children with inadequate nutritional intake in the form of enteral nutrition (EN) or parenteral nutrition (PN) support. Purpose - The purpose of this mixed methods study performed at University of Virginia Health System (UVAHS) was to evaluate the nutritional management of pediatric oncology patients using a case study approach, and to identify the barriers to providing optimal nutrition support for the three selected patients. Methods- Data collection began in February 2013 and included the selection of specific pediatric cancer patients who received EN and PN support. A total of 3 patients were selected by the UVAHS Pediatric Oncology RD. Selection criterion for the hospitalized patients were based on the following: a) UVAHS pediatric oncology patient and b) patients receiving long-term nutritional support via EN or PN feedings. The mixed methods case series involved quantitative data collection through the UVAHS EPIC electronic medical record system. Quantitative data collection identifies barriers to providing optimal nutrition support due to complications of symptom management which may arise from treatments the patients are receiving and also the 2 slow initiation of nutrition support. Qualitative research was also conducted through interviews with various members of the pediatric oncology health care team. Results - The quantitative data obtained from our study identified the importance of choosing the appropriate method for providing nutrition support whether via TPN versus EN. Improved weight maintenance was demonstrated by the quantitative data collection for a patient receiving a g-tube placement prophylactically prior to treatment. The qualitative data identified inconsistent initiation of nutrition support, further supporting the benefit of protocols regarding the initiating and advancing adequate nutrition support. Each of the health care professionals identified the lack of protocols regarding the initiation of nutrition support as a barrier to initiating and optimizing nutritional support delivery. The data from our study suggests nutrition management is not consistently being carried out per those guidelines despite timely and repeated RD recommendations. The outpatient clinic was identified as an area where nutrition management is lacking, and that the presence of the RD in clinic would be beneficial. Conclusion - Ultimately the quantitative and qualitative together identify a pattern of insufficient nutritional support provided to the three patients reviewed for the case series study. The findings from the qualitative and quantitative results suggest the need for quality improvement in providing optimal nutritional support for our pediatric oncology patients at UVAHS Children’s Hospital, potentially through the establishment of nutrition support protocols. 3 INTRODUCTION According to a 2007 study by the National Cancer Institute, an estimated 10,400 children under the age 15 were diagnosed with cancer and about 1,545 children died from the disease in the United States. These statistics make cancer the leading cause of death by disease among children 1 to 14 years of age in the United States. 1 Cancer is a disease in which the cells begin to multiply uncontrollably. Abnormal production of cells results in loss of control during growth, absence of differentiation, attack of native tissues, and the possibility of metastasis. Cancer can develop in one or more tissues or organs.1 Healthy division of DNA within cells requires tumor suppressor genes to help slow the cell growth. However, in the division of cancerous cells the tumor suppressor genes are turned off, and the cells cannot detect uncharacteristic defects. Therefore, the DNA is left vulnerable for alterations or damages in the cell structures.1 Pediatric oncology patients are susceptible to malnutrition due to rapid weight loss, which can lead to a poor prognosis. The implementation of timely and adequate nutritional support when indicated is important in promoting optimal nutrition status and quality of life for patients. Nutritional support is often given to the children with inadequate nutritional intake in the form of enteral nutrition (EN) or parenteral nutrition (PN) support 1. The enteral route is typically preferred as it is the safest way for the provision of nutrients in children with an undamaged and functional gastrointestinal (GI) tract, and prevents intestinal atrophy, toxicity, and complications of intravenous infusion.1 However, there are few randomized prospective clinical trials exploring the benefits and consequences of nutritional support for the pediatric population, and further research is needed to establish guidelines for the optimal implementation and efficacy of nutritional interventions.1 This mixed methods study performed at University of 4 Virginia Health System (UVAHS) evaluated the nutritional management of the pediatric oncology population using a case study approach in order to identify the barriers to providing optimal nutrition support for pediatric oncology patients. 5 LITERATURE REVIEW Overview of Cancer and Treatments in Pediatric Patients The leading cause of death for children under the age of 15 years is pediatric cancer. It is estimated that 11,630 children in the United States under the age of 15 years old will be diagnosed with cancer in 2013.42 An estimated 1,310 children are expected to die from cancer in 2013. 42 One third of the deaths will represent children with leukemia.42 Fortunately, the event free survival rate for children who have received a cancer diagnosis is nearing 75 percent, and the overall mortality rates for children with cancer has declined by 68% in the last four decades. 3,42 Most Common Pediatric Cancers The most prevalent cancers affecting children are leukemia, central nervous system (CNS) tumors and lymphoma.4-5 Leukemia is the most common pediatric cancer accounting for approximately 28-30% of total cancer diagnoses.4, 6 Brain and CNS tumors are the next most prevalent pediatric cancer, followed by lymphoma.4-7 An overview of the incidence of the most common forms of cancers in children can be found in Figure 1. Leukemia is a cancer in the blood-forming tissues of the body.7 There are two variations of leukemia: acute lymphatic leukemia (ALL) and acute myelogenous leukemia (AML). ALL is a cancer of the blood and bone marrow in which too many bone marrow stem cells develop into white blood cells called lymphocytes.8 Most treatment plans utilize chemotherapy in the fight against ALL but some cases also warrant the use of radiotherapy.8 ALL has an 83.8% five year survival rate. 5AML is the most common form of acute leukemia accounting for 15% of all childhood cancers.9 Modern induction chemotherapy is used to treat AML and results in a remission rate of 50-90%.5 AML has a 56.7 % five-year survival rate.5 6 Brain tumors are the largest group of solid tumors in children.7 Medulloblastoma is a brain tumor that begins formation in the cerebellum and then metastasizes via the cerebrospinal fluid 11. Medulloblastoma is the most common embryonic brain tumor representing 20% of intracranial neoplasms in the pediatric population, and mainly affects children younger than 15.11 Current treatment for medulloblastoma consists of surgical resection followed by a course of radiotherapy, craniospinal radiotherapy, and chemotherapy.11 Brain tumors such as medulloblastoma have an 85% five year survival rate.41. Lymphoma, another type of blood cancer, is most common in children below the age of 15 years. There are two different types of lymphoma: Hodgkin’s (HL) and non-Hodgkin’s lymphoma (NHL).12 HL is a highly curable malignancy, which can be cured with radiation therapy, chemotherapy, or a combination of both.13 NHL is the infiltration of malignant B cells or T cells in the lymph system and is also treated through the use of radiation, chemotherapy, or both, depending on the disease type and stage. 14 Radiation is highly effective when used to treat lymphoma, resulting in a 96% survival rate, which is the highest survival rate among all pediatric cancers.10 7 Figure 1: Childhood Cancer Incidence Rates. 5 Common Cancer Treatments in Pediatrics Pediatric cancer requires a multimodal treatment approach to provide effective treatment for the patient.2 The most frequently occurring methods of treatment are surgery, radiation, chemotherapy, and hematopoietic stem cell transplantation (HCST).10 However, these treatments can often nutritionally deplete the patient. Intensive courses of treatment can place the child at risk for acute malnutrition due to their limited nutrient stores and increased demands for growth.2 Often, the treatments for pediatric cancer can cause side effects that inhibit adequate nutritional intake. Common side effects associated with cancer treatments may include diarrhea, nausea, vomiting, mucositis, pain, fatigue, early satiety, xerostomia, and loss of taste.2 Chemotherapy: Chemotherapy is used in the treatment of cancer by inhibiting DNA synthesis of both the normal tissues and the malignant cells present in the child.16 Common side effects from chemotherapy treatment can disrupt the nutritional management of the pediatric 8 patient due to nausea and vomiting, mucositis, hepatic insufficiency, renal failure, fluid retention, diarrhea, flu-like syndrome, dehydration, ileus, constipation, hypertriglyceridemia, xerostomia, pancreatitis, and hypercalcemia.16 However, the severity of the side effects depend upon the precise medication, dosage, duration, rate of metabolism, and the predisposition of the child. 16 Furthermore, the use of chemotherapeutic agents in children can lead to malabsorption and changes made to the gut flora, which ultimately may result in unintended weight loss or frequent diarrhea.16 Table 1 below reviews the nutritional implications of the common chemotherapeutic agents. 9 Table 1. Common Chemotherapeutic Agents and their Nutritional Implications17 Drug Antitumor Spectrum Nutritional Implications Cisplatin Testicular and other germ cell tumors, brain tumors, osteosarcoma, neuroblastoma Nausea and vomiting, renal insufficiency Cyclophosphamide Lymphomas, leukemia, sarcomas, neuroblastoma Nausea and vomiting, fluid retention Cytarabine Leukemia, lymphomas Nausea and vomiting, mucositis, flu-like syndrome Methotrexate Leukemia, lymphomas, osteosarcoma Mild mucositis, hepatic insufficiency, renal failure ALL, AML, Lymphomas, most solid tumors Nausea and vomiting, mucositis, diarrhea Etoposide ALL, AML, lymphomas, neuroblastoma, sarcoma, brain tumors Vincristine ALL, lymphomas, most solid tumors Nausea and vomiting, mucositis, diarrhea, Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH), constipation Alkylating Agents: Antimetabolites: Antitumor Antibiotics: Doxorubicin Plant Product: Radiation Therapy: Radiation therapy can be used in combination with surgery and chemotherapeutic agents as part of an overall treatment plan.16 Radiation therapy works by destroying the genes of malignant cells along with the abnormal tissues that are replicating. 16 10 Ultimately the cancerous cells are killed by the radiation treatment and the tumorous mass will shrink.16 Radiation therapy can have deleterious side effects on the child depending on the region of the body radiated, dose, fractionation, length of time, and field size of the radiation administered as well as the use of other antitumor therapies, and the overall initial nutritional status of the child.16 Table 2 demonstrates the common nutrition-related implications in relation to the location of the radiation therapy provided. 11 Table 2: Nutritional Implications of Radiation Therapy and the Nutritional Side Effects17 Radiation Site Nutritional Side Effects Central nervous system: Brain and Spinal Cord Head and neck: Tongue, Larynx, Pharynx, Oropharynx, Tonsils, Salivary Glands Abdomen and Pelvis: GI Tract, Reproductive Organs, Rectum, Colon, Testicles Total body radiation: Anorexia Nausea and vomiting Fatigue Alterations in pituitary functions Growth Failure Nausea Sore mouth and throat Mucositis, esophagitis Fatigue Loss of appetite Altered taste and smell Tooth decay Altered salivation Dysphagia Nausea and vomiting Diarrhea Steatorrhea and malabsorption Fluid and electrolyte imbalances Abdominal Cramping Bloating Gas Enteritis and Colitis Lactose Intolerance Fatigue Loss of appetite Nausea, vomiting, diarrhea Mucositis, esophagitis Altered taste acuity and salivation Anorexia Delayed growth and development Surgery: Surgery, which is most often used as a part of the treatment plan to obtain a biopsy and then for possible removal of the tumor or organ affected by the cancer, results in additional complications impacting nutritional status.10 The complications that often arise as a result of surgical treatment can include insufficient oral energy and protein intake. Surgical 12 treatment may result in the inability to absorb nutrients efficiently depending upon the location of the resected tumor.16 Postoperative complications that can arise from the removal of tumors located in the GI tract or intraperitoneal organ surgery may result in impaired swallowing function, the deterioration of gastric emptying, and an ileus of the small bowel.17 The nutritional status of the child can determine the overall outcome of treatment for surgical recovery. A wellnourished child prior to surgery will have fewer surgical complications compared to those with preoperative malnourished status. The child with a preoperative malnourished status will have difficulty with improved wound healing and complications could result in increased risk for morbidity and mortality.17 Effects of Malnutrition on Cancer: Outcomes, Survival, and Recurrence Malnutrition in cancer patients is associated with a poor prognosis, and weight loss is an important predictor of mortality.18 Malnutrition discovered at diagnosis exists in between 6% to 50% of the patients and is dependent upon the histology, stage of disease, and location of the cancer.17 The side effects of malnutrition may include tissue wasting, anorexia, weakness, anemia, hypoalbuminemia, and skeletal muscle atrophy. 17 Malnutrition that is associated with protein and calorie malnutrition is associated with a higher incidence of infection, decreased tolerance of chemotherapeutic treatments, and overall diminished quality of life.17 Malnutrition has also been linked with delaying therapy which ultimately increases a child’s risk for relapse.18 All patients who are battling cancer should undergo nutritional screening to identify the presence of malnutrition and to determine the proper method of nutritional intervention. Physiology of Cancer Cachexia and Its Impact on Nutrition Status Cancer cachexia is a syndrome where the body is unable to absorb, metabolize, and utilize essential nutrients, manifesting in unintentional weight loss.19 The pathophysiology of 13 cachexia has not been clearly defined, but may be caused by the outcomes of immunologic and humoral neuroendocrine abnormalities. The physiological stress caused by cachexia can alter body composition and cause metabolic alterations in carbohydrate, lipid, and protein absorption and metabolism.17 Alterations in absorption of macronutrients will lead to weight loss due to the wasting of muscle and adipose tissues, which can induce inadequate intake and anorexia.17 Cytokines, produced by the cells of the immune system, control the feeling of satiety and gastric emptying in children. Children with cancer have higher concentrations of cytokines, contributing to the nutritional implications of cancer cachexia.17 Laboratory investigations determined the release of cytokines during cachexia is similar to the stimulatory or inhibitory effects of neuropeptides.18 Leptin is secreted by the adipose tissue which stimulates the release of anorexigenic compounds and inhibits the release of an appetite stimulant known as neuropeptide Y.18 An abnormal presence of serum leptin has been found in children with cancer.18 Table 3 reviews the role of cytokines with the response of metabolic hormones during the process of cancer cachexia. Table 3: The Role of Cytokines and Hormones in Cancer Cachexia17 Cytokine Tumor Necrosis Factor Interleukin-1 Interleukin-6 Interferon Peptide YY Ghrelin Effect on Nutrition Suppresses lipoprotein lipase, increases corticotropinreleasing hormone which suppresses food intake Blocks neuropeptide Y induced feeding; increases corticotropin releasing hormone Induces cachexia and acute phase proteins in animal models Induces cachexia; INF-g antibodies reversed wasting Levels increased with greater disease burden; inverse correlation with BMI Low values associated with greater disease burden 14 Pediatric oncology patients can experience significant metabolic changes as a result of cancer cachexia that may result in growth failure including altered metabolism and negative energy balance.18 Alterations in metabolism due to cancer can affect each of the three macronutrients: carbohydrates, proteins, and lipids.19 The changes associated with carbohydrate metabolism can include the process of glucose uptake and lactate production by the tumor, hypoinsulinism, and insulin resistance. Protein metabolism is affected by the tumor burden by altering uptake of amino acids. There may also be an association with decreased protein synthesis in muscle tissue as protein turnover is increased and protein synthesis is decreased in pediatric oncology patients; this effect may vary throughout the course of the child’s disease.1,18 Lipid metabolism is often less affected than the other macronutrients. However, the depletion of fat stores is higher in pediatric oncology patients due to increased lipolysis.1 Furthermore, children with pediatric cancers have increased resting energy expenditure which may lead to the presence of cachexia. Some research suggests that the change in basal metabolic rate is related to increased protein synthesis during certain stages of their cancer.18 Figure 2 further presents the pathophysiology of cancer cachexia. 15 Figure 2: The Pathophysiology of Cancer Cachexia44 Cancer Chemotherapy Anorexia Malabsorption Food intake Lean Body Mass & Fat Mass Pro Cachectic Factors Proteolysis Lipolysis Weight Loss Cachexia Risk of Malnutrition during Cancer Treatment Malnutrition is a common complication in the medical management of the pediatric oncology patient that affects the patient’s energy levels and overall quality of life.20 Approximately 40-80% of children become malnourished during their cancer treatments due to the aggressive nature of the treatment protocols and their negative side effects.17 A child with poor nutritional status is at higher risk for decreased immune function, delayed wound healing, and disturbances in the metabolism of drug therapies; it can also lead to poor tolerance of the therapy.1,10 A retrospective study of 455 children with different malignancies was conducted utilizing the weight-to-height ratio prior to receiving therapy. The study concluded that the patients had <80% of age-adjusted decreased significantly for survival after treatment. Children with a 16 localized disease had a difference of 30% for the survival of well-nourished patients post 36 months after treatment. The patients that had a compromised state of nutrition prior to the implementation of treatment had a significant association with relapse post therapeutic treatment for patients with solid tumors.20 Malnutrition: Definition and Assessment Malnutrition is the pathological state that results from inadequate nutrition and includes under nutrition, over nutrition, and deficiencies of micronutrients. Protein and energy malnutrition is an example of under nutrition, and is the most common form of malnutrition associated with cancer patients.5 Malnutrition often presents itself as tissue wasting, anorexia, weakness, anemia, hypoalbuminemia, and skeletal muscle atrophy.5 Children with cancer should be screened for nutritional risk at initial diagnosis and followed closely throughout treatment for the prevention of protein and energy malnutrition.5 The pediatric patient’s weight, length or height for age, head circumference, and body mass index should be assessed using the appropriate World Health Organization (WHO) growth chart for infants less than 2 years of age and the National Center for Health Statistics / Center for Disease Control (NCHS/CDC) growth chart for those children older than 2 years. The WHO recommends the weight-for-height index to assess the nutritional status of children and adolescents.1 The Waterlow criteria should be used to determine the child’s level of malnutrition using the following equation:28 % standard= actual weight measure/ expected weight measure (50th percentile) 17 The percent standard can then be utilized to assess the degree of acute or chronic malnutrition. Table 4 below reviews the standards for classifying a child with mild, moderate, or severe malnutrition using the Waterlow criteria. Table 4: Waterlow criteria for assessing nutrition status in pediatric patients18 Acute Malnutrition Chronic Malnutrition (Current weight/50th percentile (Current height/50th percentile weight for height) height for age) >0.90 >0.95 Stage 0 Stage 1 (mild malnutrition) 0.80-0.90 0.90-0.95 Stage 2 (moderate malnutrition) Stage 3 (severe malnutrition) 0.70-0.79 0.85-0.89 <0.70 <0.85 Furthermore, physical signs of malnutrition can be identified during a nutrition-focused physical exam. Table 5 demonstrates the current physical signs to identify when assessing the child’s nutritional status. 18 Table 5: Clinical Assessment of Malnutrition in Children: Physical Signs of Nutrient Deficiencies18 Physical Signs Nutrient Deficiency General appearance: edema, muscle wasting, decreased subcutaneous fat, growth failure Hair: dull, dry, may be thin and sparse, easily pluckable, color changes Eyes: Pale and dry membranes, or red and inflamed membranes, Bitot’s spots, corneal xerosis (dull and hazy) or scarring, cracking in the corner of the eyes Lips: Redness and swelling, angular stomatitis Protein, calories Tongue: Swelling (glossitis), raw tongue, magenta or purplish in color, smooth tongue, sores Teeth and Gums: May be missing teeth, bad color and visible decay or cavities, spongy, swollen and bleeding gums, recession of gums Cardiovascular system: tachycardia, enlarged heart, abdominal heart rate or rhythm GI System: spleen or liver enlargement, GI dysfunction Riboflavin, niacin Protein, calories Vitamin A, iron, folate Niacin, riboflavin, vitamin B 6 Fluoride, Vitamin C Potassium, selenium, phosphorus, thiamine Protein, calories Nutrition Side Effects and Recommendations for Management Overcoming the nutrition-related side effects of cancer treatment is a crucial step in preventing the development or worsening of malnutrition. As previously mentioned, common treatment-related side effects include nausea, vomiting, anorexia, mucositis, diarrhea, and dysguesia.18 An overview of the most common side effects is reviewed below, and Table 6 offers nutritional strategies for combating the various symptoms associated with the treatmentrelated side effects of pediatric cancer. 19 Nausea and Vomiting: Chemotherapy-induced nausea and vomiting can cause significant effects on achieving adequate nutritional intake. Uncontrolled nausea and vomiting can cause the patient to experience complications such as dehydration, electrolyte imbalance, and physical complications.21 The medical team should ensure management of the nausea and vomiting by implementing a prophylactic antiemetic regimen for every cycle of chemotherapy.21 The common treatments for the management of nausea and vomiting can include the antiemetic medications of Promethazine, Ondansetron, and Metoclopramide.5 Eating smaller more frequent meals, avoidance of overly sweet or high fat foods, avoiding unpleasant cooking odors, encouragement of drinking or sipping liquids frequently throughout the day, consumption of high carbohydrate foods and fluids, and finally avoidance of offering favorite foods during periods of nausea as this may cause permanent dislike of the food are ways to combat the inhibition of adequate nutritional intake during episodes of nausea and vomiting.5 Anorexia: Anorexia is the loss of appetite or inability to eat. Many cancer patients experience this prior to diagnosis and during treatment of the disease.22 The loss of appetite in patients with cancer-related anorexia can be due to a number of possible side effects of chemotherapy treatments.23 A proposed cause of anorexia during chemotherapy treatment is believed to be related to the effects of inflammatory cytokines on the hypothalamus that causes changes in the balance of neurotransmitters, resulting in the stimulating or inhibition of food intake. A healthy appetite relies on the balance of the appetite stimulating neurotransmitters leptin and ghrelin; leptin inhibits appetite whereas ghrelin simulates appetite.19,23 Eating six times a day, finishing what you started eating, “power packing” foods by adding extra cheese, gravy, margarine, or sauce so that each bite counts, and consuming oral nutrition supplements are ways to combat anorexia and improve nutritional intake.23 20 Mucositis: Mucositis is the inflammation of the oral mucosa that can develop subsequently after the patient receives chemotherapy treatment.25 Swelling of the tissues can cause the patient to experience painful mouth sores that can inhibit their nutritional intake and can lead to development of infections in the mouth.25 Oral mucositis can be treated with analgesics to reduce pain, and nutritional supplements can be provided to optimize caloric intake when inhibited. 25 Patients with mucositis should avoid spicy, acidic, and hard foods, and hot food/beverages due to the potential of irritation.25 Parenteral nutrition may be indicated if a patient is unable to receive enteral nutrition due to severe inflammation, lesioning, ulceration and bleeding in the oral cavity, esophagus, and lower GI tract.25 Diarrhea: Chemotherapy-induced diarrhea is a common side effect of cancer treatment and is defined as the passing of three or more liquid stools per day.42 Chemotherapy damages dividing cells that line the interior of the digestive tract and leads to disruption of fluid balance in the cells, as the absorption of fluid from the GI tract into the body is decreased but fluid in the stool is increased.42 Diarrhea can be severe enough to result in fluid and electrolyte losses that can potentially cause life-threatening dehydration, electrolyte imbalances, and renal insufficiency.42 Diarrhea is associated with an increased risk of infectious complications in children experiencing neutropenia.42 Children experiencing diarrhea during their chemotherapy treatment may need to be placed on a probiotic treatment. A standard dose of loperamide is often given to children experiencing diarrhea along with avoidance of high fat or dairy containing foods to resolve diarrhea episodes. 42 Dysgeusia: Dysgeusia is the altered sensation of taste and can be unpleasant for the child.26 The papillae in the oral cavity contain taste buds that are lined with taste receptors, which are the only epithelial cells that use neurotransmitters to transmit taste sensations to the 21 nerve fibers. Chemotherapy works to rapidly kill the division of cancerous cells but it can affect the normal healthy cells in the oral cavity as well. 26 This damage to the taste receptors results in dysgeusia. Patients often describe the taste changes as metallic, bitter, or a lack of taste during consumption of foods.26 Patients experiencing the symptoms of dysgeusia will have disruptions in appetite and digestion.26 Table 6: Nutrition Strategies for Symptom Management18 Symptoms: Nausea/Vomiting Anorexia Mucositis Diarrhea Dysgeusia Dietary Intervention Strategies: Small frequent meals, high carbohydrate content, non-acidic beverages, cold clear foods and beverages, avoid extreme temperatures and highly seasoned items, avoid high-fat content items. Small frequent meals, nutrient-dense foods and supplements, carbohydrate and protein modular, create a pleasant atmosphere, dine with the child, and vary colors/flavors/textures of foods. Soft diet, smooth bland moist foods, frozen slushes/ice/ice cream, high calorie liquid beverages Low fat, cold, or room temperature foods, avoid caffeine, encourage adequate fluid intake Herbs, spices, and marinades, cold non-odorous foods, fruit-flavored beverages, good oral hygiene, mint mouthwashes, lemon-flavored beverages and sour candies Some less common complications that arise from various cancer treatments may also lead to comprised nutritional status and may require nutrition support, including constipation, gastritis and typhlitis.27 Determining Nutritional Needs During Cancer Treatment The primary goal for the nutrition management of children and adolescents undergoing cancer treatment is to sustain and promote normal growth and development.26 All patient care providers play a key role in assuring that the necessary nutritional care is provided as part of the 22 overall treatment plan so that optimal outcomes for every child, adolescent, or young adult diagnosed with cancer can be reached. Determining nutritional needs of the pediatric oncology patient should be influenced by the following considerations: providing adequate nutrition for the preservation of lean tissue and to promote normal growth and development, identifying and correcting the effects of protein and energy malnutrition and metabolic abnormalities, and maximizing quality of life for the patient.16 Determining Nutritional Requirements The nutrient needs for pediatric oncology patients are difficult to assess because they are affected by the nutritional status, disease state, and therapy protocols. Energy requirements should be based upon the child’s age, weight, gender, therapy, and growth needs.16 Children with cancer should have their caloric needs estimated using the Basal Metabolic Rate (BMR) multiplied by an appropriate stress factor. Protein requirements are increased in children with cancer to minimize the loss of lean body mass and promote tissue repair.18 Table 7 below provides the basal energy needs for children. At the very minimum, even in critically ill states requiring intensive care unit admission, children with cancer should receive a minimum of their basal energy needs. However, in general to optimize growth and outcomes, energy and protein needs should be optimized well above the basal energy requirements. Table 8 provides the recommended estimated nutrient needs for a child with cancer. 23 Table 7: Table Basal Energy Needs for Infants and Children 28 24 Table 8: Nutrient Requirements During Childhood Cancer28 Nutrient Requirements Calories Recommendations Infants: Birth to 12 months: Use RDA for age for appropriate weight infants. Use catch-up growth calculation if underweight: (kcal/kg/day=kcal/kg/day for weight age x ideal weight age (kg) / actual weight in kg) Older children (>1 year): Use BMR table multiplied by additional factors: a) b) c) d) Appropriate weight for height: BMR x 1.6 Obese: BMR x 1.3 Sedentary with 5% weight loss: BMR x 1.4-1.6 10% weight loss from usual weight or weight is 90% or less of usual or ideal weight: BMR x 1.8-2.0 Use adjusted weight calculation for obese children; BMI weight at the 75th percentile may also be used to calculate energy needs in obese children HSCT: BMR x 1.6 during immediate post-transplant course; BMR x 1.4 following engraftment and medically stable. Infants birth to 6 months: 3 g/kg/day Infants 6 to 12 months: 2.5-3 g/kg/day Children: 2-2.5 g/kg/day Adolescents with increased lean body mass: 1.5-1.8 g/kg/day Fat 10-30% total calories Fluid 1-10 kg: 100 mL/kg/day 11-20 kg: 1000 mL plus 50 mL for every kg > 10 per day 21-40 kg: 1500 mL plus 20 mL for every kg > 20 per day > 40kg: 1500 mL/m^2 body surface area Protein 25 In addition, when epithelial cells are damaged in the GI tract due to radiation therapy, surgical removal of localized tumors, and long-term use of antibiotics, this may further increase the needs for vitamin and minerals in the pediatric oncology patient.16 However, at this time, recommendations for vitamin and mineral intake specifically geared towards the pediatric oncology patient have not been determined and current practice should be based upon the Dietary Reference Intakes.16 An age specific multivitamin with mineral supplement is recommended.16 The use of corticosteroids treatment in the children diagnosed with ALL and NHL that developed complications from HSCT such as Graft-Versus-Host disease places the child at higher risk for osteopenia and fractures.16 Children undergoing HCST may also require additional vitamin C due to the need for tissue recovery with collagen biosynthesis.16 Table 9 presents the nutrient requirements that are needed during childhood cancer. Table 9: Nutrient Requirements During Childhood Cancer28 Vitamins Minerals and electrolytes Use ASPEN parenteral vitamin guidelines for age After PN discontinued, oral multiple vitamin/mineral without iron, during antineoplastic therapy Provide additional vitamin C during HCT: a) < 31 kg, additional 250 mg vitamin C per day b) > 31 kg, additional 500 mg vitamin C per day Iron supplementation contraindicated during oncologic therapy and HCT Eliminate copper and manganese from PN in presence of hepatic dysfunction (ie, serum bilirubin > 10.0 mg/dl Closely monitor serum electrolytes during therapy 26 Approach for Nutritional Management in Pediatric Cancer Patients The Academy of Nutrition and Dietetics (AND) and the American Society for Parenteral and Enteral Nutrition (ASPEN) have described the nutrition care process in depth.18 This process contains four steps: nutrition assessment, nutrition diagnosis, nutrition intervention, and nutrition monitoring and evaluation.18 There is a clear link between screening and identification of nutrition risk.15 Nutrition assessment is of the utmost importance to helping to design optimal nutritional interventions. 15 The 2002 ASPEN guidelines state “Patients with cancer are at nutrition risk and should undergo nutrition screening to identify those who require formal nutritional assessment with development of a nutrition care plan.”29 The pediatric oncology registered dietitian (RD) plays a vital role in the nutritional management of the patient. The RD is an important member of the interdisciplinary team that includes physicians, registered nurses, speech language pathologists, occupational therapists, and physical therapists.29 The RDs at UVAHS have developed the Pediatric Nutrition Standards of Care for pediatric cancer patients. The pediatric RD must screen the patient for the nutritional risk, and nutrition assessments should include a food and nutrition related history that incorporates a 24-hour recall, hospital intake/output records, 3-day food log, or calorie count.5 Appendix A provides detailed outline of pertinent information that must be collected for the food and nutrition related history. In addition to the food and nutrition-related history, the pediatric RD must obtain the anthropometric measurements to better assess the child’s nutritional status. Key anthropometrics include length or height, weight, weight change, body mass index, and growth pattern indices/percentile ranks.5 As previously mentioned, the 2006 WHO growth curves should be 27 used for children <24 months of age and NCHS/CDC curves for > 24 months of age.5 Recumbent length should be used until 2 years of age, and then standing heights should be utilized for children above 2 years of age.5 The Waterlow criterion is currently used at UVAHS to assess the nutritional status in the oncology pediatric patient.28 The Pediatric Nutrition Standards of Care give standards for the collection of biochemical data, medical tests, and procedures.28 The RD should evaluate the basic metabolic panel, magnesium, and phosphorus for patients on nutritional support.28 Additionally, pediatric patients with increased GI losses, alterations in absorption, or individuals with potential tumor lysis syndrome should have their renal profile assessed, in addition to a comprehensive metabolic panel.28 Liver function tests should be used to evaluate hepatic function for patients on nutritional support that are at higher risk for toxic chemotherapy or medication regimens.15 Patients on PN support should have their serum triglycerides checked as well.15 Additionally, blood glucose and endocrine profiles should be checked for patients on steroid therapy with elevated serum blood glucose.15 Finally, serum levels for C-reactive protein can be used in conjunction with prealbumin as an indicator for disease acuity, and should be used to assess the inflammatory process.15 Using the information gleaned from the nutrition assessment, the RD is able to determine an appropriate nutrition-related diagnosis that will ultimately drive their recommendations for intervention. Common diagnoses for the pediatric oncology patient population are presented in Table 10. 28 Table 10: UVA Health System Common Diagnoses28 Problem Intake Inadequate protein-energy intake (5.3) Clinical Unintended weight loss (3.2) Etiology Signs and Symptoms EN or PN infusion not yet optimized Infusion providing ___ % of goal Mismatch between energy needs and energy intake, decreased ability to consume sufficient energy Anthropometric measurements, parenteral report of weight loss or report of change in usual intake Early intervention by the pediatric RD is important for the effective management of nutrition issues in cancer patients.17 RDs are available as a resource to the child and the family to explain the implications of cancer and its treatment on nutritional status.18 Moreover, the RD can work with the patient and family to help overcome the nutrition-related side effects of the patient’s treatment regimen, as previously discussed. If oral intake is inadequate, the RD is responsible for developing appropriate nutrition support recommendations to better meet the child’s nutritional needs. Nutrition Support in the Pediatric Oncology Patient An important component for the medical treatment of pediatric oncology patients is utilizing nutrition support when appropriately indicated.15 The goal of nutrition support is to provide adequate nutrients to meet the demands of the growth and development, and also to reverse the possibility of protein-calorie malnutrition. The use of nutritional support for patients that are malnourished with inadequate oral intake will improve nutritional intake and outcomes.15 If the patient is unable to meet their needs orally, nutrition support may be provided via EN or PN.15 The type of nutrition support initiated should be based upon the specific needs and conditions of the patient. 29 Enteral Nutrition Support Enteral nutrition support is nourishment given through a tube or stoma directly into the GI tract.15 EN can be administered via a nasogastric, nasojejunal, or percutaneous endoscopy gastrotomy tube.17 The benefits of utilizing EN in the pediatric oncology patient includes protecting the gut integrity and preventing bacteria translocation. In patients with a functioning GI tract, EN is preferred over PN because of the proven efficacy while decreasing the risk of infection for the child.17 The use of EN in cancer patients has been shown to improve their appetite and energy intake, as well as improve their nutritional status. EN has also demonstrated a notable reduction in GI toxicity from cancer therapies due to a better response from treatment.31,41-42 EN has also been associated with decreased risk of infections and liver abnormalities, as well as maintenance of the gut mucosa.18 Formula selection and method of administration should be individualized based on individual patient tolerance. In general, the lower osmolality in unflavored enteral formulas is better tolerated among pediatric cancer patients and should be used as the source of EN. Furthermore, due to lactose-intolerance resulting from chemotherapy, some patients may benefit from a lactose-free product that is predigested or elemental.18 Feeding schedules utilized in the clinical setting may include the use of continuous infusion, an intermittent bolus, or a combination of both.18 Children experiencing GI intolerances may benefit from continuous feeds which may improve gastric emptying and reduce nausea, vomiting, abdominal cramping, and diarrhea.18 Continuous EN may be initiated with a full strength formula at a rate of 1-2 milliliters per kilograms per hour (mL/kg/hr) and advanced 30 by 1-2 mL/kg/hr as tolerated by the child.18 Intermittent bolus feeds provide a feeding schedule that closely resembles the physiology of normal feeding, and provide children with the benefit of not always having to be hooked up to the feeding pump throughout the day.18 Finally, research has shown that a child with recurrent vomiting may benefit from post-pyloric feedings.18 Parenteral Nutrition Support Parenteral nutrition is the delivery of nutrients intravenously. The parenteral fluids usually consist of physiologic saline solution with dextrose, amino acids, electrolytes, vitamins, trace elements and medications.17, 5 The benefits of including PN in the medical management of a pediatric patient can include reversal of protein energy malnutrition, restoration of immunecompetence, and tolerance to anti-neoplastic therapies.25 However, the use of PN is associated with a higher risk for complications including line infections, hyperglycemia, hypertriglyceridemia, and cholestasis.5 PN is indicated for use if the pediatric patients are unable to meet adequate nutritional needs via oral diet or enteral feeds.5 PN may be used if a child has an immature GI tract, or an intestinal obstruction. Furthermore, patients that have had a surgical resection of a tumor located in the bowel may have malabsorption of nutrients and may necessitate the provision of nutrition intravenously.5 Finally, in cases of malnutrition, PN may be used if chemotherapy or radiation therapy delays the use of EN .5 PN support can be administered through peripheral or central venous access.32 The peripheral vascular access requires several site changes. Dextrose solutions used with peripheral PN are limited to a concentration of 10% to 12.5% and are difficult to meet the high caloric demands of the pediatric oncology patient.32 The risk of phlebitis, inflammation of the vein, is higher with peripheral access.17 Central venous access allows for the dextrose to have a higher 31 concentration to the limit of 35% with amino acids at 6 %.32 However, patients on PN support require more vigorous metabolic monitoring than those on EN support.17 Enteral Nutrition Support versus Parenteral Nutrition Support Research supports the overall benefits of EN in pediatric oncology patients and validates that PN should only be used as a nutritional intervention when EN is not indicated.33 Unfortunately, the use of EN is inconsistent in the pediatric oncology population despite its known benefits when compared to the use of PN. One survey determined that EN was rarely routinely administered prior to the initiation of PN.18,33,35 The side effects associated with intensive chemotherapy treatment such as mucositis often discourages the use of EN feeds. In addition, children may have neutropenia or thrombocytopenia that can increase their risk for bleeding when the tube is inserted; however, clinical trials have not supported this theoretical risks.18 PN is traditionally the preferred method among children receiving myeloablative conditioning for HSCT.33,35 However, studies show that EN is well tolerated in this population as well, with 77% of patients requiring no PN during the post-transplant period.35 Furthermore early placement of a percutaneous endoscopic gastrostomy (PEG) for EN has been shown to eliminate the risks of lesions in the GI mucosa.6 Early placement of the PEG tube would allow for the provision of adequate nutrients via EN throughout the transplant process, positively impacting nutritional status and decreasing risk of infection or tube dislodgement due to vomiting when compared to PN and EN through a nasogastric tube.36 Clinical trials in children with cancer have found EN to be a more cost effective and safe approach compared to PN, even in the settings of high-dose chemotherapy and bone marrow 32 transplantation.18 One study showed that body weight increased significantly in the intervention group receiving EN compared with the control group who received PN, and all of the children in the intervention group reached their ideal weight compared with only 21% in the control group.1 Moreover, PN can be costly for the patient in comparison to the use of EN, and also significantly increases the patient’s risk of infection. One study showed that the monthly costs associated with the use of gastrostomy nutritional support were 9% of those costs associated with total PN .33-34 Furthermore, the risk of sepsis in children receiving PN is 2.4-times greater compared to children with central venous access but that do not receive PN .37,38 Barriers to Optimal Nutrition Support in Pediatric Cancer Barriers to providing adequate nutrition per the recommendations of the RD include initial low infusion rates, feeding interruptions due to medical procedures, and holding feeds for specific medication administration.40 To overcome this, careful monitoring and established protocols utilizing higher calorie formulas and increased tube feeding volume are recommended to compensate for the interruptions.40 Furthermore, the potential side effects related to EN may act as barriers to providing optimal nutritional support in the pediatric patient.39 Patients that experience frequent vomiting will benefit from antiemetic therapy, reductions in volume, and the use of promotility agents.40 The development and validation of uniform nutrition protocols will assist in creating consistency in the provision of optimal nutrition support among institutions treating children with cancer.18 With a review of standards of practice for nutritional support in pediatric cancer patients and common barriers, we will now investigate current practice and nutritional management for cancer patients at UVA Children’s Hospital. 33 Current Practice at UVA Children’s Hospital The most common pediatric cancer seen at UVAHS is childhood leukemia which accounts for 33% of all pediatric cancers. According to the UVAHS pediatric oncology nurse practitioner, approximately 75% of patients seen at UVA with leukemia have ALL, and the other 25% have AML. Cancer treatments are dependent upon the individualized patient’s diagnosis and the severity and stage of the disease, but commonly include chemotherapy, radiation, and/or surgical intervention. The chemotherapy agents most often used are vincristine, l-asparaginase, methotrexate, cyclophosphamide, cytarabine, ifosfamide, etoposide, doxorubicin and cisplatin. The pediatric oncology RD at UVAHS follows the oncology patients closely while in the inpatient units, using the guidelines set forth by the Pediatric Oncology Standards of Care as previously described. Unfortunately, current practice for the implementation of timely and appropriate nutrition support has been inconsistent among this patient population. 34 PURPOSE The purpose of this case series based mixed methods research project was to investigate the barriers to providing hospitalized pediatric oncology patients with optimum nutritional support at UVAHS. The specific goals of this study were to: Determine barriers to optimizing nutrition support Investigate the medical team’s preference for use of a particular form of nutrition support. Provide evidenced-based recommendations for the nutritional management of pediatric oncology patients Promote the importance of utilizing EN feedings as a standard nutritional intervention during treatment. Recommend the revision and active implementation of the pediatric nutrition standards of care for the pediatric cancer population through review and approval by the Pediatric Hematology Oncology Team at UVAHS Promotion of improved nutritional management and outcomes for pediatric oncology patients during hospitalization. 35 MIXED METHODS CASE SERIES METHODOLOGY This study used a case series mixed methods approach to investigate nutrition support practice management at UVA Health System and to determine the association between the implementation of nutritional support via EN or PN feedings and nutritional outcomes and tolerance in three selected hospitalized pediatric patients. Data collection began in February 2013 and included the selection of specific pediatric cancer patients who received EN support. The patients were selected by the UVAHS Pediatric Oncology RD. Selection criterion for the hospitalized patients were based on the following: a) UVAHS pediatric oncology patient and b) patients receiving long-term nutritional support via EN or PN feedings. A total of three patients were selected for this case series review. Quantitative Data The Epic electronic medical record (EMR) system utilized at UVAHS was used to retrospectively collect data on the three selected patients. Initial data collected on each patient included specific cancer diagnoses, reason for current admission, previous surgical and medical history, anthropometrics including weight (kg), length (cm), head circumference (cm), oncologic treatments received, and the degree of malnutrition assessed by the Waterlow Criteria. During each admission nutritional intake data including PN order, actual intake, and caloric intake (kcal/kg) from PN was obtained, if applicable. Additionally, EN intake data was obtained to include specific formula and caloric density, feeding schedule and route, actual 24 hour intake, and caloric intake (kcal/kg) from EN. Total caloric intake from both PN and EN was recorded as well as the patient’s nutritional goals and percentage of the goals achieved throughout the 36 admission. Additional information regarding the barriers to starting nutritional support, percent weight loss, and relevant medications were collected. All of the collected data was recorded and organized into a Microsoft Excel spreadsheet as presented in Appendix B. The quantitative data obtained through this study was used to provide a general overview of the outcomes and current nutritional practices at UVAHS. This mixed method case series study provided a general overview on the use of EN versus PN and the management course for pediatric cancer patients at UVAHS. Qualitative Data Qualitative data collection included the RD’s recommendations for adequate provision of calories and protein and information gleaned through interviews with various members of the pediatric oncology health care team. Three specific healthcare professionals were interviewed including a pediatric oncology bedside nurse, pediatric oncology nurse practitioner and the pediatric oncology RD at UVAHS. The interview questions for the various healthcare professionals are presented in Table 11-13. The qualitative data obtained through this study was also used to provide a general overview of the outcomes and current nutritional practices at UVAHS and provided a general overview on the use of EN versus PN and the management course in pediatric cancer patients at UVAHS. 37 Table 11: Interview Questions with the Pediatric Oncology NP Table 12: Interview Questions with the Bedside RN 38 Table 13: Interview Questions with the Pediatric RD 39 RESULTS Introduction and Patient Demographics This case series review study included three selected hospitalized pediatric patients. As previously mentioned, the data collection was completed from February 2013 until the end of March 2013 and consisted of the type of nutritional support provided, the formula order, and actual intake of the nutritional support. The three chosen pediatric oncology patients presented with varying degrees of malnutrition, in need of nutrition support, and were appropriate candidates for the use of the GI tract. An overview of the demographic data, weight changes and degree of malnutrition for each of the patients is summarized below. Furthermore, Table 14 summarizes each patient’s diagnosis, weight and height changes and degree of malnutrition at their various admission dates. Patient 1 The first case series patient is T.W., a 7 year old male with a history of anaplastic medulloblastoma. For the purposes of this paper, data collection for T.W. began upon his first presentation to UVAHS in which the current pediatric oncology RD assumed patient care in August 2011. The patient’s weight upon the first admission used for data collection was 18.8 kg, consistent with moderate malnutrition per Waterlow criteria. During this admission, T.W. received a course of chemotherapy treatments consisting of cyclophosphamide and viscristine. T.W. had several subsequent admissions due to complaints of decreased appetite, abdominal pain, and worsening nausea. Additionally, he experienced varying levels of malnutrition and weight fluctuations throughout his treatment, with his lowest weight of 17.8 kg in January of 2012 (Stage 2 moderate malnutrition). T.W. had a gastrostomy tube placed in February 2012, 40 which resulted in significant improvements in weight status initially. However, T.W. continued to experience abdominal pain, nausea and vomiting which contributed to poor feeding intolerance and weight loss despite enteral access. In July 2012, T.W. presented to UVA Children’s Hospital with complaints of a 5 day history of worsening headaches, nausea, and vomiting. A brain MRI showed slight increase in ventricle size and more prominent leptomeningeal enhancement suggesting a progression of his metastatic disease. Neurosurgery was consulted due to the results of the MRI and a ventriculoperitoneal shunt (VP) was placed to treat intracranial pressures secondary to hydrocephalus in hopes of relieving some of the patient’s symptoms. Unfortunately, the VP shunt did not provide much relief and patient returned to PICU with decreased responsiveness. The pediatric palliative care team was consulted on July 25th, 2012 and made recommendations for comfort care. T.W. expired on July 29th, 2012 during his final admission at UVA Children’s Hospital. Patient 2 The second case series patient presented is N.P., an 11 month old male first admitted to UVA Children’s Hospital for infantile AML. The patient has a past medical history of a full-term infant with Bell’s palsy and history of pertussis. N.P. first presented to Pediatric Intensive Care Unit (PICU) at UVAHS on August 14th, 2012 in critical condition due to persistent fluid overload from new onset AML and acute kidney injury. His admission weight was 9.2 kg (21st %-ile), and at the time of initial presentation was well-nourished (Stage 0 per Waterlow Criteria). The patient had PN initiated upon admission. EN was started September 2nd, 2012 due to impaired intake status-post anterior cricoid split for subglottic stenosis and he was weaned off of TPN and lipids. N.P. continued to require NG feeds upon transfer to the floor on September 21, 2012. During the course of admission on the floor N.P. transitioned to daytime bolus feeds but 41 due to improved PO intake the physician decided to discontinue bolus feeds to allow for continued progress with PO intake. On September 30th, 2012, N.P. was transferred back to the PICU due to increased work of breathing and found to have recurrence in leukemia cutis. His prolonged hospitalization continued and during that time received a combination of radiation therapy and chemotherapy. However, due to disease progression, poor tolerance to feeds due to anesthesia prior to daily radiation, and the development of typhlitis during his intensive radiation and chemotherapy regimen, N.P. required PN prior to his transfer to Duke University Hospital for radiation therapy and evaluation for cord blood transplant. Unfortunately, due to disease progression and complications with leukemia cutis, N.P. was not a candidate for transplantation and returned to UVAHS for comfort care. At 15 months old the patient expired on December 12th, 2012 at UVA Children’s Hospital. Patient 3 The third patient studied in our case series is N.M., a 7 year old female diagnosed with medulloblastoma of the cerebellum in July 2012. N.M. underwent surgical resection of her tumor, followed by a course of radiation therapy. Due to the side effects of radiation and significant weight loss of 15 pounds of body weight during radiation treatment, the pediatric hematology and oncology team decided to pursue gastrostomy tube placement in October 2012, anticipating worsening malnutrition once chemotherapy was initiated. The gastrostomy tube was placed at the same time as her central line, just prior to her initial admission for chemotherapy. During October 2012 upon admission N.M. was well-nourished per Waterlow Criteria though at risk related to history of 17% severe loss of body weight. Upon admission N.M. had regained 100% of weight back as compared to the usual body weight of 67 pounds. EN was initiated via nocturnal feeds to meet 50% of needs with the remainder of nutritional needs to be met with 42 regular diet during the day. During admission of January 2013, N.M. presented for chemotherapy of maintenance cycle two with treatments of vincristine and cyclophosphamide. It was noted during the admission that her weight had improved from 26 kg to 29.1 kg subsequently following the placement of the G-tube. However during a second admission due to typhlitis in January 18th, 2013, N.M. was found to have a 9% weight loss in 2 weeks, classified as moderate malnutrition. N.M. continues to receive chemotherapy treatments and nocturnal feeds via her G-tube. She presented to UVAHS Children’s Hospital on February 1st, 2013 with an admission weight of 26.6 kg which was decreased from her January admission weight of 29.1 kg. N.M. presented with symptoms of feeding intolerance with g-tube feedings, severe abdominal pain, and emesis. During the admission of March 4th, 2013, N.M. was considered to be well-nourished per Waterlow criteria with a weight of 25.2 kg. However, N.M. was considered to be at nutrition risk during this admission due to 13% weight loss in a month and half. N.M. is currently undergoing ongoing chemotherapy treatments, and continues to receiving nocturnal feeds via her G-tube. 43 Table 14: Patient Demographics Patient 1 – T.W. Gender (M/F)* M DOB 4/29/04 Age: 7 Age: 7 Age: 7 Age: 7 Age: 8 Initial Diagnosis Medulloblastoma (2010) Weight (kg) (%-ile) Height (cm) (%-ile) Degree of Malnutrition± (Waterlow Criteria) 18.8 kg (3.5th%ile) 120 (26th%ile) 1-8/12/2011 19.5 kg (<5th %ile) 120 (26th%ile) 120 (26th%ile) 119.6 (8th%ile) 122.5 (10th%ile) 68 cm (0.09th %ile) 81 cm (73%ile) Stage 2 (Moderate Malnutrition) Stage 2 (Moderate Malnutrition) Stage 2 (Moderate Malnutrition) Stage 2 (Moderate Malnutrition) Stage 2 (Moderate Malnutrition) Stage 0 (well-nourished) Stage 0 (well-nourished) Stage 0 (well-nourished) Stage 0 (well-nourished) Stage 0 (well-nourished) Stage 0 (well-nourished) Stage 0 (well-nourished) *moderate malnutrition given weight loss of 9% in 2 weeks Stage 0 (well-nourished) *moderate malnutrition given 13% weight loss in 1-10/3/2012 21.5 kg (8th%ile) 22.5 kg (10tth%ile) 21.6 kg (8th%ile) 2 -N.P. 3 N.M. M F 9/04/11 Age: 13 months Age:15 months Infantile AML (2012) 5/30/05 Age: 7 years Medulloblastoma (2012) Age: 8 years 9.96 kg (45%%ile) 10.7 kg (60th%ile) 24.2 kg (55th%ile) 26 kg(69%ile) 30.9 kg (90th%ile) 29.1 kg (82%ile) 26.6 kg (67%ile) 25.2 kg (53th%ile) 127cm (72nd%ile) 127cm (72nd%ile) 127cm (72nd%ile) 129.5cm (75th%ile) 129.5cm (75th%ile) 128cm (65th%ile) Total Admissions Reported And Dates 2-2/09/2012 3-3/14/2012 4-4/18/2012 5-7/24/2012 1-10/12/2012 2-12/10/2012 2- 10/25/2012 3- 12/07/2012 4- 1/18/2013 5-2/01/2013 6-3/04/2013 one month Nutrition Support Course, Management and Outcomes The approach to nutritional management of these three patients will now be presented. Each patient’s nutritional management course, RD recommendations, and nutrition support order by the physician is presented in Tables 15-17. Patient 1 For the first patient, T.W., with medulloblastoma three separate sections will be presented, representing each of the three admissions as presented in Table 15. As can be gleaned from this patient’s admission in August of 2011, despite this patient’s poor nutritional status, history of weight loss and poor appetite, and the pediatric RD’s recommendations for initiating nutrition support, he was only nutritionally managed with a Pediatric Regular diet and supplements. T.W. was referred to clinical nutrition on December 21st, 2011, due to poor weight gain and previous use of NG feeds during previous admission at John Hopkins with supplementation of Peptamen Jr. 1.5. During the admission of December 21st, 2011, the patient had a weight of 19.5 kg with a weight-for-age at the 3.45 percentile. T.W. had been following a home nutrition regimen of 8 cans of Peptamen Jr. 1.5 mixed into a pitcher with whole milk and chocolate ovaltine; he would and consumes this volume within 2 days. The family was incorporating ways to improve caloric intake by increasing caloric value of foods. However upon admission January 13th, 2012, T.W. had a 2 pound weight loss over the preceding 3 weeks. T.W. had been taken off of Megace during this time and experienced a decrease in appetite. The oncology team decided that given the patient’s recent significant weight loss that it was indefinite to place an NG tube for improved long-term weight maintenance given 46 appetite stimulants were not facilitating hunger and would only be a short term fix. The patient’s weight had fluctuated over the last year and half and improved significantly following the placement of a G-tube in February 2012 with a 2.8 kg weight gain. T.W. was admitted to UVAHS again on April 18, 2012. Throughout the course of the 3 day admission, despite the pediatric RD’s recommendations, EN was not started to provide optimal nutrition support until the second day of admission due to frequent episodes of emesis despite the 1 kg loss prior to admission (5 % loss of body weight). The RD recommendations included initiating Peptamen Jr with 3.5 cans to be supplied by nocturnal EN via NG feeds to meet 75% of nutritional needs and to allow for PO intake during the day and the patient received full feeds as recorded by actual intake. However, during the admission of April 2012, the intake records demonstrated the patient was only consuming 25% of PO diet with overnight EN feeds meeting 100% of estimated EN goal. During the patient’s 5th admission to UVAHS on July 24th 2012, the patient presented with worsening headache, nausea, and vomiting secondary to tumor progression. The patient’s weight at the time of admission was 21.55 kg which equates for weight-for-age at the 8th percentile. T.W. had lost 0.9 kg from his April 2012 admission and was 90% of his IBW. Nutritional support management was not initiated and patient was kept on a NPO diet based on recommendations from the palliative care team for comfort care measures only. 47 Table 15. Nutritional Management Review for Patient #1 – Patient with Medulloblastoma (T.W.) RD Nutrition Nutrition Nutrition Encounter Order Goals Recommendations Admission #1: 8/12/2011; Reason for Admission: Medulloblastoma Medications Comments 1 – 8/12/2011 Peds Regular Ensure TID D5% ½ NS with KCl 75-80 kcal/kg 1.5-2 gm pro/kg 75-80 ml/kg 1.Start TPN: Dextrose 15% and 20 gram of protein/day to goal of 35-40 mL/hr; Lipids 20% 100 mL/day (1 g/kg lipid) Marinol Ondansetron Cyclophospha mide Patient admitted with poor appetite, weight loss, and abdominal pain. Patient began on Marinol. BMI was <5th%-ile. Estimated intake during this time was 40 kcal/kg or 50% of needs or approximately half of needs. TPN not initiated although recommendations made 2-08/15/2011 Peds Regular Ensure TID D5% ½ NS with KCl 75-80 kcal/kg, 1.5-2.0 g pro/kg, 75-80 mL/kg 1. Begin TPN: Dextrose 15% and 20 gram of protein/day to goal of 3540 mL/hr; Lipids 20% 100 mL/day (1 g/kg lipid) Marinol Ondansetron Cyclophospha mide 3-08/23/2011 Peds Regular Ensure TID D5% ½ NS with KCl 80 kcal/kg, 1.5-2.0 g pro/kg, 80 mL/kg 1.Begin TPN : Dextrose 15% and 20 gram of protein/day to goal of 3540 mL/hr;Lipids 20% 100 mL/day (1 g/kg lipid) PhosNak, Potassium Chloride, Ondansetron Patient is 76% of IBW (Moderate Malnutrition per Waterlow) Reported poor PO intake>4 days Frequent episodes of emesis Reports Marinol is not helping appetite Acute hepatic failure, respiratory distress due to fluid overload TPN not initiated although recommendations made Developed central line infection, fever, diarrhea Unable to take NPO given emesis. TPN not initiated during admission although recommendations made due to line infection NPO for Bone Marrow Procedure Found to have veno-occlusive disease of liver Admission # 2: Add Date 02/08/2012; Reason for Admission: Chemotherapy, further management of disease progression, and increased pain. 102/09/2012 Peds Regular Ensure TID Peptamen JR 1.5 @ 70 mL/hr over 12 hours D5% ½ NS with KCl 85-95 kcal/kg for weight regain, 2g protein/kg 77 mL/kg 1.Begin EN: 3.5 cans of Peptamen JR 1.5 @ 70 ml/hr over 12 hours to provide: 45ml/kg, 67 kcal/kg, 2.0 g pro/kg. Morphine, Lorazepam, Periactin, Colace, Miralax, Ondansetron IBW: 22.5 kg and weight is currently 18.7 kg which is 83% of IBW Little weight gain over the last 15 months with weight significantly below 5% weight for age Considered mild chronic malnutrition given weight loss Nocturnal NG feeds will meet 75% of caloric/protein needs and allow PO intake during day Actual Intake: 67 kcal/kg, 2.0 g pro/kg. 45ml/kg on February 9th, 2012 Admission # 3: Add Date 03/12/2012; Reason for Admission: Chemotherapy and cellulitis surrounding G-tube stoma 1.Continue Methadone, ondansetron, IBW: 22.5 kg and weight is currently 20.6 kg which is 92% of providing IBW. lorazepam, clindamycin Pediatric diet. Appetite improved while on steroids but declined as steroids 2. Continue were discontinued home G-tube Weight up ~2kg in a month since the placement of the G-tube. regimen as tolerated: 3 cans of Peptamen Jr. 1.5 70 mL/hours over 10hours; Provides:52 kcal/kg, 1.6 kg pro/kg 2.Encourage PO intake with snacks and supplements PRN (ex. Ensure Plus) Admission # 4: 4/18/2012; Reason for admission: Abdominal pain and erythema surrounding G tube site, fever, emesis 1-03/14/2012 Peds Regular Ensure TID D5% ½ NS with KCl 85-95 kcal/kg for weight regain, 2 g pro/kg, 77 mL/kg 1-4/18/2012 NPO 80-90+ kcal/kg, 2 g pro/kg, 76 mL/kg 1.Begin TPN if unable to resume EN: Dextrose 10%, 40 grams of protein, 1 g/kg of 20% Intralipids. 2. Increase dextrose to 15% and then to goal of 18%. Final concentration of lipids 2 g/kg. 3. Resume PO diet with additional Methadone, Dilaudid, ondansteron, decadron, lorazepam, famotidine, and antibiotics IBW of 22.5 kg and 87% of IBW with 1 kg loss in the past month (5% body weight) Started Marinol about week ½ ago and seems to stimulate appetite. Recommendations made but diet not advanced due to complications of cellulitis, peritoneal infection and began to tolerate PO intake on 04/20/2012 Ensure Plus TID and home EN regimen as medically feasible: Peptamen Jr 1.5 with 3 cans over 10 hour period @ 80 mL/hr to meet 60% of estimated needs. 2-4/23/2012 Peptamen Jr 1.5 Nocturnal feeds over 10 hr, Regular pediatric diet 80-90+ kcal/kg, 2 g pro/kg, 76 L/kg 1. Continue PO diet as tolerated; encourage PO intake 2. Chocolate Ensure Plus ordered TID 3. Continue Nocturnal feeds as tolerated. Would dilute somewhat given emesis and the fact the patient has not reached goal feeds with Peptamen 1.5 at home yet. Mix: 560 mL Peptamen Jr. 1.5 with 160 mL water, run at 75 ml/hr over 10 hours; would be equivalent to 1 can of Peptamen Jr. 1.5 + 2 cans Antibiotics, IVF Ondansteron, Methadone, Morphine PCA, Lorazepam, Dexamethasone, Famotidine < 25% consumed of PO diet Frequent N/V and believes this is related to the enteral formula. Mother believed it was due to formula change of Peptamen Jr to Peptamen Jr 1.5.; Feeds were held Patient weight is currently 19.3 kg with an IBW of 22.5 kg. T.W. is currently 87% of IBW. Weight improved since the G-tube placement of February 9th, 2012. T.W. previous weight before G-tube placement was 18.7 kg with 83% of IBW of 22.5 kg. Patient weight was 20.6 kg and 92% of IBW of 22.5 kg Actual Intake: 75 kcal/kg, 2.2 g pro/kg, 75 ml/kg Meeting 94 % kcal, 100% protein of estimated EN nutritional goals with minimal PO intake within 24 hours. Peptamen Jr 1.0 (44 kcal/kg, 1.3 g/kg protein, meeting ~50% of estimated needs) Peptamen Jr 80-90+ 1.Plan for Antibiotics, Metadone, 1.5 kcal/kg, discharge with dexamethasone, IBW of 22.5 kg and is 87% of IBW. Nocturnal 2.0 g pro/kg, home regimen of famotidine, lorazepam, Frequent emesis feeds over 76 mL/kg 3 cans of ondansetron, drobinol Actual Intake: 60 kcal/kg, 1.8 g pro/kg, 60 ml/kg 10 hrs; Peptamen Jr 1.5 Meeting 75% kcal, 90% protein of EN nutritional goals within Regular the last 24 hours pediatric diet Admission # 5:07/24/2012; Reason for admission: Worsened headache, nausea, vomiting secondary to tumor progression 3-4/26/2012 1– 7/24/2012 NPO 80-90 kcal/kg, 1.5-2 g pro/kg, 71 mL/kg 1.Peptamen Jr. 1.0 (2 cans) + Peptamen Jr. 1.5 (1 can) at 75 mL/hr over 10 hrs to provide nocturnal feeds. 2. If not tolerating PO diet initiate Peptamen 1.5 (5 cans) at 75 mL/hr continuous Zofran, Decadron, IVF, Pepcid, Miralax, Morphine PCA, Ativan Weight fluctuated over the last year and half. Improved weight since G-tube placement in Feb. 2012 BMI has improved. IBW of 24 kg and is 90% of IBW Declined PO > 3 days, and persistent emesis. Diet unable to be advanced 2-7/26/2012 NPO 80-90 kcal/kg, 1.5-2 g pro/kg, 71 mL/kg 1.Advance from NPO diet to clear liquid diet 2.Provide Resource Breeze nutritional supplement TID 3. Continue to advance diet as tolerated and clinically feasible 4.If unable to advance diet then initiate 5.Pediasure via G-tube at 10-15 mL/hr and advance Ancef x 3, Zofran, Decadron, Miralax, Pepcid Transferred to PICU following VP shunt placement. Weight tracking between the 5th and 10th percentiles on the CDC growth curve charts since 6.5 years Enteral nutrition not provided due to Palliative Care recommendations. Patient remained on NPO diet. 3- 07/29/2012 NPO 80-90 kcal/kg, 1.5-2 g pro/kg, 71 mL/kg by 10 mL every 6 hours to goal of 65 mL/hr No recommendations made Morphine PCA, Fentanyl PCA, Ativan Pediatric Palliative Care consulted on 7/25/2012 Recommendations for pain control and comfort care Patient 2: However, N.P. experienced weight fluctuations towards the end of his prolonged admission, as his full nutritional goals were not met consistently due to inadequate oral intake coupled with insufficient EN delivery, due to feeding interruptions due to the radiation schedule, anesthesia, and typhilits. As can be gleaned from this infant’s admissions, Patient 2 was managed primarily with EN. During that admission, N.P. had been weaned off of TPN and lipids and transitioned to EN feeds of Pediasure (Abbott Labs, Columbus, OH). At the start of radiation therapy, N.P. weighed 9.86 kg (47%ile weight for age), demonstrating adequate weight gain of approximately 10 grams per day over the previous 2 months of admission due to EN support. During the course of radiation, N.P. EN feeds were scheduled to be stopped at midnight while receiving radiation, further posing a barrier to suboptimal nutrition support via EN feeds. N.P. was transitioned to bolus feeds to optimize nutrition given limitations of his treatment schedule, although he did have some problems tolerating afternoon bolus feeds related to post-anesthesia effects. He was also placed on a pureed diet with nectar thick liquids during the day based on the recommendations of the speech language pathologists. N.P. was transitioned back to nocturnal feeds on November 11th, 2012 after completing radiation therapy, in an effort to improve tolerance to EN feeds and to improve PO intake during the day. The patient’s weight trended up to 10.17 kg (50% weight for age) on November 12th, 2012 and was tolerating current nutritional regimen. However, on November 15th, 2012, the patient’s EN was placed on hold after increased emesis and abdominal x-ray concerning for right lower quadrant pneumatosis. N.P. was started on TPN and lipids and advanced with trial of a clear liquid diet with Pedialyte (Abbott Labs, Columbus, OH) provided via NG tube. 53 The patient was subsequently transferred to Duke Medical Center for a bone marrow transplant. However on December 8th, 2012, N.P. presented to UVA Children’s Hospital from Duke Medical Center due to the rapid progression of leukemic involvement throughout multiple organ systems. N.P. was considered to no longer be a viable candidate for transplantation and preceded with the services of palliative care. N.P. expired on December 12, 2012. 54 Table 16. Nutritional Management Review for Patient #2 – Patient with Infantile AML (N.P.) RD Encounter Nutrition Order Nutrition Goals Nutrition Recommendations Medication Comments Admission #1: 08/08/2012; AML worsened by Pseudomonas tracheitis 1 – 10/15/12 2-10/18/2012 Pediasure 1.0 overnight at 40 ml/hr over 12 hours via NG D5% ½ NS with 20 mEq KCl/L Propofol 90105kcal/kg 2.5-3 gm pro/kg 100 ml/kg NPO for radiation Pediasure 1.0 with fiber 160 mL q 3-4 hrs x 6 daily feeds + Pureed tray with Nectar thick liquids 90-105 kcal/kg, 2.5-3 g/kg pro, 100 mL/kg 1. Increase bolus feeds to 135 ml x 7 daily feeds as tolerated to optimize nutrition with radiation schedule.Provides: 4 cans of Pediasure 1.0 (97 kcal/kg, 2.9 g protein/kg) 2. As clinical feasible, resume PO intake per SLP recommendations. Offer oral pediasure with remaining volume via NGT as PRN. Encourage solids ad lib 3. When intake of solids resume, begin strict calorie count Ondansetron Dexamethason e Cytarabine, 1. Continue PO diet per SLP recommendations as tolerated and clinically feasible; encourage PO intake Stomatitis Cocktail, Famotidine, Cytarabine, Methadone, Miralax, Ativan 2. Continue Bolus regimen of 160 ml q Transferred from the PICU on enteral nutrition via NGT for overnight feeds and daytime bolus. Previously receiving TPN and lipids and advanced to continuous EN to overnight with PO ad lib of baby/soft foods with additional provided supplements of Pediasure, Carnation Instant Breakfast, and Boost Breeze while in the PICU Actual Intake:76 kcal/kg, 2.2 g protein/kg, 76 ml/kg Meeting currently 76 % of estimated enteral nutrition goal Emesis associated with suctioning of trach and hyper gag reflex Starting radiation M, T, Th, F through 10/28/12 Had been transitioned to bolus feeding regimen due to feeding interruptions from radiation schedule 3 day average via NGT 10/15-10/17: 43 kcal/kg, 1.3 g/kg protein, meeting ~43% of estimated needs. SLP working with patient on PO intake; recommended 3-4 hours over 6 feeds per day Purees and Nectar thick liquids 3. Calorie count to assess adequacy of PO intake and ability to decrease NG feeds 4. Nutrition discharge planning: n/a at this time 3-10/25/2012 Dysphagia 1 Pediasure 1.0 with fiber 160 mL q 3-4 hours x 6 daily feeds 90-105 kcal/kg, 2.5-3 g/kg pro, ~100 mL/kg 1. Transition back to overnight continuous EN regimen as tolerated and as medically feasible given last scheduled day of radiation. Hopefully will allow for improved tolerance, as well as better meet his needs more evenly throughout the course of the day, and promote increased PO intake. Goal Regimen as follows: PO diet ad lib per SLP recommend ations; goal ~500 kcal/day PO Daytime bolus feeds only PRN with Pediasure 1.0 enteral with fiber: Stomatitis Cocktail, Famotidine, Cytarabine, Zofran, Dexamethason e, Etoposide 3 day average: 48 kcal/kg, 1.4 g/kg pro, and 25-50% PO intake via meals Continues on NG bolus feeds during the day to optimize nutrition with radiation schedule. Actual Intake: 69 kcal/kg, 2.0 g protein/kg Meeting 72% of estimated EN nutrient needs. 4-11/01/2012 Pediasure 1.0 with fiber Nocturnal Dysphagia 1 diet with pureed foods and nectar thickened fluids. 90-105 kcal/kg, 2.5-3 g/kg pro, ~100 mL/kg 160 ml at 10am, 2 pm, or 6pm; use only as replacement if patient refuses a meal, otherwise allow PO ad lib Continuous overnight EN of Pediasure 1.0 at 50 mL/hour over 10 hours Calorie count to assess PO adequacy to attempt to decrease NGT feeds 1.Providing clears (pedialye). If ongoing bouts of emesis, will consider IV fluid support when NPO to provide ½ MIVF. 2.Begin EN: Pediasure 1.0 with fiber Nocutrnal:500mL, Refuses meals than PRN daytime bolus feeds 160 mL at 10 am, 2 pm, and 6 pm Colace, Antibiotics, Stomatitis Cocktail, Famotidine, Cytarabine, Methadone, Zofran, Dexamethason e, Etoposide 3 day average: 483 mL, 48 kcal/kg, 1.4 g/kg pro, and 2550% PO intake via meals MD held EN overnight given large volume emesis in the morning associated during chemotherapy Calorie Count (3 day average): 106 kcal/kg; 2.5 g protein/kg; 57% PO intake Actual Intake: 69 kcal/kg, 1.8 g protein/kg Currently meeting 72% of estimated EN nutrient needs. 5- 11/08/2012 6-11/15/2012 Pediasure 1.0 with fiber Nocturnal: 50 mL/hr x 8 hrs Dysphagia 1; PO ad lib 90-105 kcal/kg, 2.5-3.0 g/kg pro, ~100 mL/kg NPO TPN: Dextrose 15% 88 kcal/kg, 2.8 g/kg of pro Encourage daytime PO intake but continue nocturnal continuous feeds. 1.Continue PO diet ad lib per SLP recommendations; goal 600 kcal/day PO 2. Continue EN nocturnal feeds with Pediasure 1.0 at 50 ml/hr over 8 hours 3. Would consider daytime bolus feeds PRN due to decreased PO and weight Bolus Feeds PRN: Pediasure 1.0 of 160 ml at 10 am 2pm, and 6 pm If not able to restart bolus than increase nocturnal feeds to 55 ml/hr over 9 hours or consider Pediasure 1.5 1.Begin TPN: Dextrose 15% 30 g/day Trophamine Stomatitis Cocktail, Famotidine, Cytarabine, Zofran, Dexamethason e, Etoposide Pepcid, Stomatitis Cocktail, Patient continues to have episodes of emesis 3x/day with suctioning and refuses PO intake. Calorie Count (3 day average): 63 kcal/kg1.2 g protein/kg, Meeting 39% of needs of PO intake Actual Intake: 31 kcal/kg, 2.2 g protein/kg Currently meeting 77.5 % of EN estimated nutrient needs Patient made NPO to decrease risk for aspiration pneumatosis; increased emesis and concern for Mucositis Actual Intake: 1,001.5 mL TPN + 153.1 mL 20% IL: 91 7-11/23/2012 8-11/27/2012 30 g/day Trophamine 155 mL NPO Dextrose 15% 30 g/day Trophamine 155 mL NPO Dextrose 15% 30 g/day Trophamine 155 mL 100 mL/kg 155 mL Zofran 90-105 kcal/kg, 2.5-3 g/kg of pro 3 g/kg IL 100 mL/kg 90-105 kcal/kg, 2.5-3.0 g/kg of pro 3 g/kg IL 100 mL/kg 1. Resume pureed diet when medically feasible 2. Continue TPN with D 15%, 30 g protein at 42 ml/hr + 3gm/kg IL. Adjust electrolytes PRN 3. As medically feasible restart NG feeds with Pediasure 1.0. Can titrate TPN/EN 1:1 Start feeds at 10 mL/hr and increase 5 ml/hr q 4 hours Goal is 42 ml/hr to meet 100% of needs Pepcid, Stomatitis Cocktail, Zofran, Peridex, 1. Continue D15%, 30 g protein +3 g IL in TPN; Cycle to 20 hours for hepatoprotection2. Resume EN when medically feasible of Peptamen Jr 1.0 at 10 ml/hr and increase 5 ml/hr q 4 hours to Colace, Pepcid, Miralax, Stomatitis Cocktail, Zofran, Peridex, kcal/kg, 2.9 g/kg of pro, 306.2 kcal Lipids Currently meeting 100% of nutritional needs Patient is neutropenic with concern for Typhlitis. MD decided due to continued vomiting and not tolerating and remove/replace NG tube. Concern for recurrence of leukemia cutis and oncology to evaluate. Actual Intake: 996.7 mL TPN + 149.7 mL 20% IL: 90 kcal/kg, 2.9 g/kg of pro, 299.4 kcal Lipids Meeting 100% of TPN nutritional needs MD put on bowel rest with TPN initiated. MD began clear liquid diet with Pedialyte 11/25 average weight gain of 8 grams/day since 11/1; meeting goal for age. 11/29 N.P. was transferred to Duke Children’s Hospital for bone marrow transplant. TPN Actual Intake: 1,045.9 mL TPN + 164.1 mL 20% IL: 95 kcal/kg, 2.9 g/kg of pro, 328.2 kcal lipids Currently meeting 100% of TPN nutritional needs goal of 42 ml/hr Admission # 2: Date; Comfort Care 1-12/10/2012 NPO TPN: Dextrose 15 %, 30 grams/day Trophamine, 161 mL/day 90-100 kcal/kg, 2.5 g/kg protein, 3 g/kg IL 97 mL/kg Continue TPN at the request of patient’s parents. Morphine PCA and Ativan Rapid progression of leukemic involvement throughout multiple organ systems. No longer a candidate for transplantation. NGT was removed on 12/08/2012 TPN Actual Intake: 45 kcal/kg, 2.8 g/kg protein, 120.8 kcal lipid/day Currently meeting 51% of TPN nutritional needs Patient 3: As can be gleaned from this child’s admissions, Patient 3 was managed with EN support with the placement of a G-tube requested by the oncologist, given the child’s severe weight loss during treatment. N.M. experienced a 17% severe weight loss within 4 months of diagnosis. Upon initial visit with the pediatric oncology RD, N.M. weighed 24.2 kg on October 3rd, 2012. N.M. was able to regain to a weight of 1.8 kg prior to gastrostomy tube placement on October 24, 2012 due to improved appetite while on steroid therapy. Following G-tube placement, N.M.’s weight continued to improve with the provision of continuous nocturnal feeds. The patient presented to UVA Children’s Hospital on December 6th, 2012 with a weight of 30.9 kg and a weight-for-age percentile of 90th. The weight during this admission had improved significantly from 26 kg documented prior to placement of the g-tube and patient had regained back to usual body weight of 30.5 kg. From October to December 2012, N.M. was following a home EN regimen of 2-4 cans of Pediasure provided via the G-tube overnight. Unfortunately, in January 2012, N.M. was admitted with complaints of intolerance with overnight feeds but improved tolerance with a bolus EN regimen. N.M. weights had trended down 1.8 kg from the previous admission weight of 29.1 kg in December to 29.1. The January admission was complicated due to concern of infection at the g-tube site during this admission due to low grade fever and pain around g-tube site. N.M. experienced intermittent emesis but not associated with the EN feeds and decreased appetite. On February 1st 2013, N.M. presented to UVAHS Children’s Hospital due to complaints of abdominal pain and frequent episodes of emesis. Her weight upon admission was 26.6 kg, demonstrating continued loss of 2.5 kg since January. This equates to a weight loss of 9% body weight within a two week period and placed N.M. at risk for moderate malnutrition given the weight loss. During the February admission, N.M. was able to tolerate nocturnal feeds once 61 again, to provide 3 cans of formula per night, meeting 55% of estimated needs via supplementary EN. At this time the pediatric RD recommended adding a fourth can as tolerated while oral intake remained poor. This recommendation was not implemented during the February admission. On March 4th, 2013, N.M. presented with a neutropenic fever and weight loss of an additional 1.4 kg from her previous weight of 26.6 kg during the February admission, placing her at risk for moderate severe acute malnutrition. The RD implemented recommendations for a gradual advance back to goal feeds. However, the RD’s recommendations for an enteral formula change were not carried out during this admission per the primary team. Currently, N.M. continues to undergo treatment for medulloblastoma. Unfortunately, a recurrence of disease was found April 2013 and primary oncologic care is now being provided at an outside hospital. She continues to receive supplementary EN via her G-tube. From October to December 2012, N.M. was following a home EN regimen of 2-4 cans of Pediasure provided via the G-tube overnight. Unfortunately, in January 2012, N.M. was admitted with complaints of intolerance with overnight feeds but improved tolerance with a bolus EN regimen. N.M. weights had trended down 1.8 kg from the previous admission weight of 29.1 kg in December to 29.1. The January admission was complicated due to concern of infection at the g-tube site during this admission due to low grade fever and pain around g-tube site. N.M. experienced intermittent emesis but not associated with the EN feeds and decreased appetite. On February 1st 2013, N.M. presented to UVAHS Children’s Hospital due to complaints of abdominal pain and frequent episodes of emesis. Her weight upon admission was 26.6 kg, demonstrating continued loss of 2.5 kg since January. This equates to a weight loss of 9% body 62 weight within a two week period and placed N.M. at risk for moderate malnutrition given the weight loss. During the February admission, N.M. was able to tolerate nocturnal feeds once again, to provide 3 cans of formula per night, meeting 55% of estimated needs via supplementary EN. At this time the pediatric RD recommended adding a fourth can as tolerated while oral intake remained poor. This recommendation was not implemented during the February admission. 63 Table 17. Nutritional Management Review for Patient # 3 – Medulloblastoma (N.M.) RD Encounter Nutrition Nutrition Nutrition Medications Comments Order Goals Recommendations Admission #1: 10/03/2012; Clinic visit, RD consulted due to severe weight loss throughout radiation therapy 1 – 10/03/2012 n/a 65-80 Zofran, Marinol 1. Continue to Significant weight loss 17% body weight in the last 3.5 months kcal/kg, (severe loss). maximize calorie and 2-2.5 g Weight is down from UBW of 65 # (29 kg) protein intake with pro/kg, Appetite is waxing and waning with small volumes being consumed. small frequent meals 62L/kg Significant frequency of N/V since diagnosis and snacks + fortifiers + nutrient dense beverages 2. Agree with consideration of gastrostomy placement; recommend starting with nocturnal feeds to help alleviate the caloric burden, with the option of daytime feeds prn Admission #1: 10/24/2012; Central line and G-tube placement and initiation of 9 cycle chemotherapy treatment 1-10/25/2012 Pediatric 65-80 1. Nutritional Decadron, Well-nourished per Waterlow criteria given weight regain due to Regular Diet kcal/kg, supplements TID and in- Zofran, steroids though at risk due to history of severe weight loss (17% body D5% NS 2-2.5 g house trial of PRN famotidine weight). with 20 mEq protein/kg, feeding regimen via G PO intake of fluids is still below goal of 50 oz/day with typical intake KCl at 30 62 mL/kg tube. The methods of of 24-30 oz/day. mL/hr continuous or bolus were discussed with family 2- 10/26/2012 D5 ½ NS 65-80 1. Begin Pediasure 1.0 Decadron, Hem/Onc and PSGY approved G Tube use with 20 mEq kcal/kg, enteral with fiber @ Zofran, Eating better today but hasn’t had a BM in 4 days and Miralax KCl at 30 2-2.5 g 15mL/hr and advance by famotidine, administered today. mL/hr, Mg protein/kg, 15 mL/hr q 6 hrs as fentanyl, Tolerated initiation of EN feeds via G-Tube. Discharged home on sulfate 8 62 mL/kg tolerated to goal of 90 miralax, nocturnal feeding regimen with daytime bolus feeds PRN. mEq at 125 mL/hr vincristine, mL/hr 2. Home regimen: cisplatin, Pediasure 1.0 @ 90 mL/hr x 10hrs overnight; provides 50% of estimated needs 3. If limited PO intake during the day, administer 3 bolus feeds/day (q 4-5 hours) prn of 300 mL/bolus Admission #3: 12/06/2012; Maintenance cycle of chemotherapy infusion 1Pediatric 50-60 1. Continue with PO 12/07/2012 Regular Diet kcal/kg , ad lib during the day 2-2.5 g + goal of 4 cans protein/kg 62 mL/kg Pediasure 1.0 lomustine Sulfate drip and miralax overnight Weight 30.9 kg (90%ile) upon admission; improved from 26 kg 10/24 when G-tube placed, BMI 90% Appetite has improved with help from steroids although now discontinued. Parents noticed decline in appetite and started g-tube again for nocturnal feeds (2-4 cans/night). 2. Supplement with Pediasure bolus(es) during the day PRN to reach goal of 7 cans/day if taking minimal PO . Admission #4: 1/17/2013; Maintenance chemotherapy cycle 2 1-01/18/2013 Pediatric 50-60 1.Resume home g-tube Regular Diet kcal/kg, regimen: 8 oz Pediasure 1.5-1.8 g 1.0 enteral with fiber, run protein/kg, at 120 mL/hr x 3 feeds 58 mL/kg per day. 2. Fluid goal of ~24-26 oz/day via PO and water flushes Admission #5: 1/31/2013; Abdominal pain and emesis Zofran, Ativan, dexamethasone, vincristine, mesna, cyclophosphami de, miralax 29.1 kg (82%ile); improved weight from 26 kg (10/24) when g-tube placed. UBW 67# (30.5 kg), BMI 79%ile, Length 129.5 cm, well-nourished per Waterlow criteria Intolerance to continuous feeds overnight and had been doing bolus regimen at home PTA. Home Regimen: 3-8 oz bottles per day of pediasure; over ~2 hours with extra 4 oz. water flushes after each bolus 02/01/2013 Pediasure 1.0 enteral with fiber Nocturnal 60 mL/hr x 12 hours Via g-tube 720 mL/day Pediatric Regular diet 55-70 kcal/kg, 1.5-1.8 g protein/kg, 60 ml/kg 1. Initiate appetite stimulant and encouragement of PO intake. 2. Nocturnal feeds of Pediasure 1.0 with fiber at 60 mL/hr x 12 hours; if minimal PO intake then add additional can via continuous feeds @ 60 mL/hr. 3.Add water flushes of 60 mL before and after each feed to provide 8 oz/day; with PO goal of at least 8-16 oz/day fluids. Antibiotics, miralax, Ativan, zofran 65-80 kcal/kg for weight regain, 1.5-1.8 g protein/kg, 65 mL/kg 1. Regular diet and encouragement of PO intake, snacks ordered BID between meals. 2. Nocturnal G-tube feeds to provide 2 cans/night at 40 mL/hr x 12 hours, advance to 3 cans/night as tolerated. 3.Consider change to peptide-based formula given persistent emesis Zofran, miralax, phosnak TID 26.6 kg (67%ile); Weight down from 29.1 kg (1/18) with 9% weight loss in the last 2 weeks. UBW 67# prior to diagnosis (30.5 kg), BMI 57%ile, moderate malnutrition given recent weight loss Overnight feeds providing 55% of enteral nutrition goals Admission #6: Neutropenic fever 103/04/2013 Pediatric Regular Diet Pediasure 1.0 with fiber @ 60 ml/hr x 12 hours overnight via G-tube 25.2 kg, well-nourished per Waterlow criteria, though at nutrition risk due to 13% weight loss in the last month and a half RD made goals for halting further weight loss and meeting >50% of nutritional needs within 48 hours Actual EN Intake: 23 kcal/kg, 0.85 g protein/kg, 23 mL/kg in previous 24 hours Meeting only 80.5% of estimated EN goals and remainder of nutritional needs provided via PO intake 203/07/2013 Pediatric Regular Diet Pediasure 1.0 with fiber Nocturnal @ 50 ml/hr x 10 hours 65-80 kcal/kg for weight regain, 1.5-1.8 g protein/kg, 65 mL/kg 1. Regular pediatric diet and encouragement of PO intake 2. Snacks ordered BID between meals. 3. Pediasure 1.0 with fiber via Nocturnal feeds @ 50 ml/hr x 10 hours, advance as tolerated to goal of 60 ml/hr x 12 hours 4. Continue to recommend consideration of peptide-based formula Zofran, miralax, phosnak TID, Frequent emesis with full feeds but has been tolerating feeds at lower rates the last two nights (30ml/hr, 40 ml/hr). Team not yet ready to switch to peptide-based formula Actual Intake: 10 kcal/kg, 0.43 g protein/kg, 10 ml/kg Meeting 50% of nocturnal EN nutrition goals with remainder of nutritional needs provided via PO diet Qualitative Data – Interviews with Health Care Professionals Introduction In order to obtain basic information on the current nutritional support practices at UVAHS, interviews were conducted with various members of the pediatric oncology health care team to include a pediatric oncology nurse practitioner (NP), pediatric oncology bedside nurse (RN), and a pediatric RD currently employed at UVAHS. The health care professionals provided their perspectives on the optimal approach and challenges in the management of pediatric oncology patients. A summary of the responses from the three healthcare professional interviews can be found in Tables –16-18. Additionally, the full transcript of the interviews can be found in Appendix C. 68 Table 18: Answer Summaries of Interview with Pediatric Oncology NP Interview Question What are the most common pediatric cancers treated at UVA Children’s Hospital? Is there a protocol or guideline that you follow regarding the nutritional management of the pediatric oncology patient? At what point do you feel nutrition support is indicated in this patient population? Answer ALL, AML , CNS brain tumors No established guidelines but follow the Children’s Oncology Group recommendations The NP personally focuses on weight loss of 510% usual body weight to determine the initiation for enteral nutrition or parenteral nutrition. UVA Children’s Hospital does not have a determined number for weight loss as a determinate initiating for nutritional support. Advantages: Eliminates burden of caloric intake, relieves tension between child and parent, electrolytes are easier to maintain with TPN, extra bolster of nutrition Disadvantages: Higher risk for infection, damaging to the liver Advantages: provide oral medication through tube, alleviate tension with child and parent, not as expensive as TPN, easier to administer. Disadvantages: “Children hate the NG tubes”, tube dislodgement during emesis, source of infection, g-tube requires surgical placement. No barriers identified Children often resistant to g-tube placement for enteral nutrition What are the advantages and disadvantages to using parenteral nutrition support? What are the advantages and disadvantages to using enteral nutrition support (whether through NGT or via GT)? What are the biggest barriers or complications associated with using nutrition support in the inpatient setting? Please note if you are referring to EN and/or PN. How do you feel nutrition management of the pediatric oncology patient could be improved? Improved patient education handouts for clinic Earlier initiation of nutritional support RD to round consistently with oncology medical team RD to follow-up with patients in the outpatient clinic RD to provide nutritional support in-service to pediatric oncology medical staff 69 Overall, as can be reviewed from the interview data, the oncology NP stated that the UVA Children’s Hospital currently has no established guidelines regarding the nutritional management of the pediatric oncology patient. The NP believed the pediatric oncology team questions their nutritional management decisions retrospectively once the pediatric oncology patient’s nutritional status has deteriorated for example: placing a g-tube sooner, different placement of tube, or started TPN earlier. Currently the NP follows the supportive guidelines provided by The Children’s Oncology group. The intervention of nutrition support should be initiated when a child has lost 5% of body weight. In addition, she made the following recommendations for improving the nutritional status of their outpatient pediatric oncology population: implementing nutritional handouts to provide to the families during outpatient visits, the appropriate timely initiation of EN, more consistent participation from the RD on medical team rounds, nutritional education for the medical team, and the overall availability of an RD in the outpatient clinic to follow-up with the pediatric oncology patients. 70 Table 19: Answer Summaries of Interview with Bedside RN Interview Question What are the advantages and disadvantages to using parenteral nutrition support? What are the advantages and disadvantages to using enteral nutrition support (whether through NGT or via GT)? At what point do you feel nutrition support is initiated in this patient population? What are the biggest barriers or complications that you see associated with using nutrition support in the inpatient setting? How do you feel nutrition management of the pediatric oncology patient could be improved? Answer Advantages: symptom management for mucositis or nausea Disadvantages: neutropenic, large fluid volumes, more susceptible to inaccurate recording of the input and out records Advantages: The ability to meet nutritional needs if the patient refuses to take all calories by mouth Disadvantages: The family and patient adapting to meeting all calorie needs via NGT/GT when the patient was previously receiving via mouth Depends on the patient and which attending is on service Depends on how the parents feel about the lack of PO intake Enteral feedings: Formula availability Parenteral feedings: no barriers identified Standard protocol not needed Earlier initiation of enteral nutrition. Overall, as can be reviewed from the interview data, the pediatric bedside nurse reported one barrier to providing EN for the pediatric oncology patients is the availability of the enteral formula in the units. However the beside RN identified no barriers to providing PN to the pediatric oncology patients. In addition, she recommended earlier initiation of EN to improve the nutritional status of their pediatric oncology population, and believed that factors contributing to initiation of nutrition support were impacted by which attending was on service and how concerned the parents were regarding lack of nutrition intake. However, she did not feel that it would be feasible to develop standard protocols a UVA Children’s Hospital for the management of nutritional support. 71 Table 20: Answer Summaries of Interview with Pediatric RD Interview Question What are the most common pediatric cancers treated at UVA Children’s Hospital? Is there a protocol or guideline that you follow regarding the nutritional management of the pediatric oncology patient? What are the most common topics of nutrition education that are covered with this patient population? Answer The Children’s Oncology Group’s algorithm for the initiation of nutrition interventions and nutrition support Maximizing nutritional intake with protein and calorie fortifiers Making the most of the calories the children are drinking How to optimize intake despite severe nausea, vomiting, or taste changes Food safety practices At what point do you feel nutrition support is indicated in this patient population? What are the factors that influence your recommendations for starting nutrition support? What are the advantages and disadvantages to using parenteral nutrition support? What are the advantages and disadvantages to using enteral nutrition support (Whether through NGT or via GT)? ALL, AML, Wilm’s Tumor, Medulloblastoma, Neuroblastoma, and Sarcomas Begin nutrition support if a patient has lost >5 of their UBW Consider upcoming treatments and anticipated side effects when making recommendations Anticipate PO intake in the interim, overall % weight loss, nutrition status prior to diagnosis to individualize when nutritional support should be initiated Family of patient may be more comfortable with the use of TPN over EN TPN is often used as a way to manage electrolyte disturbances from chemotherapy Greater risk for infection with the use of TPN and higher dextrose infusion TPN is less physiologic, doesn’t stimulate gut mucosa like EN, and does not provide the immune-stimulating benefits that using the gut can provide. EN is more physiologic and has improved benefits in terms of maintaining the gut mucosa. Substantially less expensive than TPN and has lower risks for infection Disadvantages include: tube placement which may require surgery, uncomfortable 72 What are the biggest barriers or complications associated with using nutrition support in the inpatient setting? How do you feel nutrition management of the pediatric oncology patient could be improved? nasoenteric tubes, and possible self-image issues For patients with active mucositis without prior enteral access, it’s difficult to convince the team to place a tube that might irritate the mucositis or cause bleeding in a child with thrombocytopenia. Families/patients reluctant to using NG tubes as part of their care plans. Families often see weight loss as “expected” given the nature of their child’s treatment and IV nutrition may be seen as an “easier” fix. TPN often utilized more than EN, even if EN may be indicated. “[Nutrition support] may affect quality of life, and with other issues like mucositis and n/v, unfortunately EN is often not started as proactively as it could be. Once it’s started I think the biggest barriers to optimizing nutrient delivery are 1) tolerance, and 2) feeds being held due to interruptions.” “Proactive instead of reactive efforts in starting nutrition support, particularly EN. I also think it would be helpful to have more follow-up from an RD in clinic, to allow for the continuum of care, and to be able to identify needs and adjust regimens in a timelier manner (vs. only seeing these patients when they’re inpatient).” As can be reviewed from the interview data, the pediatric RD reported that nutrition recommendations are made based on current standards provided by the Children’s Oncology Group. The guidelines recommend initiation of nutrition support if a patient has lost >5% of their Usual Body Weight (UBW). The UVAHS Children’s Hospital does not have current protocols in place for the initiation of nutritional support in pediatric oncology patients. The RD provides recommendations for nutritional support by considering upcoming treatments (chemotherapy, surgery, or radiation), anticipated side effects of the treatments, and anticipation of appetite in the interim, overall % weight loss, and nutrition status prior to diagnosis. Based on the interview 73 with the pediatric oncology RD, she feels that improvements to the nutritional management of hospitalized pediatric oncology patients could be made by initiating nutrition support earlier, and via the appropriate utilization of EN versus PN. In addition, more consistent follow up from the pediatric oncology RD in clinic may help to provide optimal nutrition support, allowing for the maintenance of the continuum of care, and to be able to identify needs and adjust regimens in a timelier manner. 74 DISCUSSION As suggested in the literature review, children with cancer have higher nutritional needs requiring adequate nutrition to be provided by their oral diet, EN, or PN. Achieving adequate nutritional intake is often difficult due to the symptoms that may arise from treatment that will inhibit the ability to adequately meet the patient’s needs via the oral route. Children with cancer are more susceptible to malnutrition due to altered metabolism and rapid weight loss. Malnutrition was noted in two of three patients used in our case study upon their initial admission of data collection as was noted in Tables 12-14. Furthermore, over the course of data collection, each of the three patients experienced mild malnutrition during a hospital admission in which patients did not have nutritional support initiated but were maintained on NPO with intravenous fluids only. The quantitative data collected within this study has shown that each of the three patients did not receive adequate nutritional support with EN during their admissions at UVAHS Children’s Hospital. Individualized nutrition care plans were recommended by the RD for each patient to receive adequate nutritional support, but patients remained NPO or on a regular diet with no additional nutritional support provided. Data also showed that when nutrition support was initiated it was not advanced to the goal rate or was often delayed due to procedures during at least one admission date recorded for each patient. According to the nutrition intervention algorithm presented in Appendix D was developed by the Children’s Oncology Group to establish guidelines for the process of nutrition support should be initiated when a patient has incurred > 5% weight loss from their usual body weight or during therapy for the treatment of their oncologic process. Additionally, a patient is identified as needing nutrition support if their weight for length has crossed > 2% ile channels. During T.W.’s admission on August12th 2011, 75 the patient was identified as having a BMI < 5%, 76% of IBW, and a weight loss > 5% of usual body weight. The physician had consulted the RD to provide recommendations for TPN. Recommendations were made by the RD to provide adequate nutrition with TPN on the first day of the admission and subsequently on a number of days following the initial assessment. Unfortunately, the nutrition intervention was never implemented during this admission due to central line infection. However as evidenced by the algorithm, nutrition intervention should have been implemented upon the first day of admission given that T.W. was at risk for moderate malnutrition. Given that T.W.’s oncologic process warrants TPN or EN intervention based upon poor PO intake > 4 days with average intake < 50% of estimated needs the patient was kept NPO due to line infection, procedures, and fluid overload.. Per the algorithm, patients who cannot meet adequate nutrition needs > 80% from an oral diet should begin on nutrition support. However the diet was not advanced from NPO due to the barriers of developing a central line infection and prior episodes of emesis during the admission. The optimal route of nutrition intervention, whether EN versus PN, is dependent upon the patient’s ability to safely tolerate and absorb nutrients into the GI tract. T.W. had a few episodes of GI intolerances due to emesis, but these GI intolerances possibly could have been alleviated by appropriate formula selection or the use of antiemetic or motility agents. If the intolerances can be eliminated by the use of antiemetic agents then the patient is a candidate for EN. Given T.W.’s development of a central line infection with the use of TPN, it only further supports the research indicating the overall benefits of EN in pediatric oncology patients and validates that PN should only be used as a nutritional intervention when EN is not indicated.33 The development of a central line infection inhibited the medical team from optimally providing adequate nutrition support for T.W. during the admission 76 of October 12th, 2011. Retrospectively looking at the data collection, it presents the issue that if there were protocols regarding the initiation of nutrition support. Would the appropriate nutrition intervention have been chosen on the first day of admission thus preventing the barrier of providing optimal nutrition support? Another barrier to providing optimal nutrition support for the pediatric oncology patient is the holding of EN for emesis and other side effects such as mucositis. For example, on August 12th, 2011, NG feedings were held due to emesis and concerns for mucositis. Given these concerns, the medical team removed the patient’s NG tube. Consequently, the patient never reached full EN feeds, with average feeds meeting only 67% of estimated EN goals. Ultimately, the patient was made NPO, further preventing the achievement of optimal nutrition support. However, prophylactic placement of a PEG tube before treatment may be beneficial to providing additional nutrition support for the pediatric oncology patients with suspected concerns for malnutrition during treatment.45 Patients often experience ulcerative oral mucositis and are likely to suffer from nausea and vomiting, thus interfering with the ability to eat or have normal swallowing function.45 The placement of the tube prior to beginning therapy in anticipation of inadequate ability to meet nutritional needs may prevent dehydration, malnutrition, and weight loss, further interrupting treatment and placing the patient at further risk for complications.45 A good example of placing a G-tube in anticipation of malnutrition exists in our third case study patient, N.M. The patient presented to UVAHS Children’s Hospital on October 3rd, 2012 with a 17% severe loss of body weight within 3.5 months since diagnosis after undergoing radiation therapy. The decision was made to place a G-tube in light of upcoming chemotherapy treatments, severe weight loss, and minimal average intake as reported by a food diary. After Gtube placement, the patient’s weight significantly improved by 4.5 kg in December 2012 to a 77 weight of 30 kg. However, without standard protocols regarding the initiation of nutrition support and appropriate nutrition intervention in place, optimal and consistent nutrition support cannot be provided for the pediatric oncology patient population. The qualitative data provided by the pediatric oncology NP, RD and bedside RN provided additional perspectives from the various healthcare providers regarding the current practices for implementing nutrition support at UVAHS Children’s Hospital. Each of the health care professionals consistently identified the lack of protocols regarding the initiation of nutrition support for the pediatric oncology patient at UVAHS as a problem and barrier to optimizing nutritional support delivery and initiation. The RD and nurse practitioner both identified the Children’s Oncology Group as a reference when providing recommendations for nutritional management for the pediatric oncology patient; however; the data from our study suggests nutrition management is not consistently being carried out per those guidelines despite timely and repeated RD recommendations The NP and RD also both identified the outpatient clinic as an area where nutrition management is lacking, and that the patients and oncology team would benefit from the presence of the RD in clinic as patients often experience side effects from their treatments that inhibit their nutritional intake. The presence of an RD available in clinic would help with symptom management and allow for proactive adjustments as needed to home nutrition support regimens. Ultimately, both the quantitative and qualitative together identify a pattern of insufficient nutritional support provided to the three patients reviewed for this case series study. The findings from the qualitative and quantitative results suggest the need for quality improvement in 78 providing optimal nutritional support for our pediatric oncology patients at UVAHS Children’s Hospital. 79 CONCLUSION AND RECOMMENDATIONS The results of the qualitative and quantitative data obtained in this mixed methods case series study further supports the need for an establishment of nutrition support protocols at UVAHS Children’s Hospital. The quantitative data indicated inadequate provision of nutrition support during the patients’ admissions. In a few circumstances, the appropriate route of nutrition support, whether EN versus TPN, was utilized in an effective manner. However, this was the exception rather than the norm. Establishment of nutrition support protocols would uniformly provide pediatric oncology healthcare professions, with guidelines for the timely initiation of appropriate nutritional support in our pediatric oncology patients. The optimization of nutritional support for this patient population would allow for the provision of adequate nutrition to support normal growth and development. Furthermore, the qualitative data collection supported the need for uniform protocols in providing nutrition support for pediatric oncology patients in the inpatient unit as well as outpatient clinic. The qualitative data further supported the RD as being a key member of the medical team and providing recommendations for nutrition support for the pediatric oncology patients. However, having the RD on the pediatric oncology team is not beneficial to patient outcomes if that individual’s timely and appropriate nutritional recommendations are not implemented. The RD is an important health care professional who provides proactive recommendations for nutritional support in a timely and effective manner based on established guidelines. The RD could play a vital role in improving the effectiveness of nutritional support by educating the healthcare staff on the role of nutrition support in the pediatric oncology patient 80 population and appropriate nutrition recommendation, particularly if an RD is unavailable in the outpatient clinics. Providing education on nutrition support to staff members will only reinforce the importance of initiating nutritional support in a timely matter for the pediatric oncology patient with increased nutritional needs. The UVAHS Dietitians have developed a Pediatric Nutrition Support Handbook “Standards of Care” that could be utilized along with the existing algorithm from the Pediatric Oncology Group presented in Appendix D to collaboratively develop nutrition support protocols for pediatric oncology inpatients admitted to UVA Health System. Furthermore, the availability of an RD in the outpatient clinic is important to having an effective medical staff to provide recommendations for nutritional support and information on symptom management in the pediatric oncology patient along the continuum of care. 81 REFERENCES 1. Bauer, J. Important Aspects of Nutrition in Children with Cancer. Journal of Advances in Nutrition. 2011; 2: 67-77. 2. Sanner, N. Acute and Chronic Nutrition Considerations in Pediatric Oncology. Topics in Clinical Nutrition. 2012; 27(3): 305-314. 3. Boklan, Jessica. Little patients, losing patience: pediatric cancer drug development. Molecular Cancer Therapuetics. 2006; 5: 1905. 4. Young, G MD, Toretsky, J MD, Campbell, A MD. Recognition of Common Childhood Malignancies. American Family Physician. 2000; 61(7): 2144-2154. 5. Marcris, P, Hunt, K. Hematology and Oncology. In: Pediatric Nutrition. 4th ed. Dallas, TX: Helm Publishing; 2010: 363-382. 6. Hawkins, C, Croul, S. Viruses and human brain tumors: cytomegalovirus enters the fray. The Journal of Clinical Investigation. 2011; 121 (10): 3831-3833. 7. Colby-Graham, M, Chordas, C. The childhood leukemias. The Journal of Pediatric Nursing. 2003; 18 (2): 87-95. 8. Torpy, J MD, Cassio L, MA; Glass, R MD. Acute Lymphoblastic Leukemia. Journal of the American Medical Association. 2009; 301 (4): 452. 9. Prakash, Balendu. Treatment of relapsed undifferentiated acute myeloid leukemia (AMLM0) with Ayurvedic therapy. International Journal of Ayurveda Research. 2011; 56-59. 10. Haddy, R MD, Haddy, T MD. Lifetime Follow-up Care After Childhood Cancer. Journal of the American Board of Family Medicine. 2010; 23 (5): 647-654. 11. Jozwiak,J, Grajkowska, W, Wlodarski P. Pathogenesis of medulloblastoma and current treatment outlook. Medical Research Reviews. 2007; 27 (6): 869-890. 12. Mantadakis, E, Herrera, L, Leavey, P, et al. Fractionated Cyclophosphamide and Etoposide for Children With Advanced or Refractory Solid Tumors: A Phase II Window Study. Journal of Clinical Oncology. 2000; 18 (13): 2576-2581. 13. de Alarcon, P MD, Arceci, R. Pediatric Hodgkin Lymphoma Treatment & Management. Medscape References Drugs, Diseases, and Procedures. 2011. 14. Myers, N RN. What you need to know about non-Hodgkin's lymphoma. Nursingcenter.com. 2009; 5 (3): 18-23. 82 15. Sacks, N, Wallace, E, Desai, S et al. Oncology, Hematopoietic Transplant, and Survivorship. In: American Society for Parenteral and Eternal Nutrition, Pediatric Nutrition Support Core Curriculum. ASPEN; 2010: 363-382. 16. Samour, P, King, K. Pediatric Nutrition. 4th ed. Sudbury, MA; Jones & Bartlett; 2012. 17. Veldhuis, J, Roemmich, J, Richmond, E, et al. Endocrine Control of Body Composition in Infancy, Childhood, and Puberty. Endocrine Reviews. 2005; 26 (1): 14. 18. Ladas E, Sacks N, Meacham L,et al. Multidisciplinary Review of Nutrition Considerations in the Pediatric Oncology Population: A Perspective From Children’s Oncology Group. Journal of Nutrition in Clinical Practice. 2005; 20: 377-393. 19. DeWys W. Pathophysiology of Cancer Cachexia: Current Understanding and Areas for Future Research. American Association for Cancer Research Journal. 1982; 42: 721-725. 20. Ward E, Hopkins M, Williams N, et al. Nutritional Problems in Children Treated for Medulloblastoma: Implications for Enteral Nutrition Support. Journal of Pediatric Blood Cancer. 2009; 53: 570-575. 21. Aseeri, M, Muhktar, A, Khansa, SA, et al. A retrospective review of antimetic use for chemotherapy induced nausea and vomiting in pediatric oncology patients at a tertiary care center. Journal of Oncology Pharmacy Practice. 2012. 22. Arensmeyer, K RN. Nursing Management of Patients with Cancer-Related Anorexia. Onco Link. 2012. 23. Dahlin, C., Lynch, M., Szmuilowicz, E., & Jackson, V. Management of symptoms other than pain. Anesthesiology Clinics of North America. 2006; 24: 39-60. 24. Fischer Walker, C, Friberg, I, Binkin, N. Scaling Up Diarrhea Prevention and Treatment Interventions: A Lives Saved Tool Analysis. PLOS Medicine. 2011; 8 (3). 25. Cheng K, Chang A, Yuen M. Prevention of Oral Mucositis in Pediatric Patients Treated with Chemotherapy: A Randomized Crossover Trial Comparing Two Protocols of Oral Care. European Journal of Cancer. 2004; 40: 1208-1216. 26. Baggott, C, Dodd, M, Kennedy, C, et al. Multiple Symptoms in Pediatric Oncology Patients: A Systematic Review. Journal of Pediatric Oncology Nursing. 2009; 26 (6): 325-329. 27. Pashankar, F, Rodriguez-Galindo, C, Pappo, A, et al. Journal of Pediatric Hematology/ Oncology. 2012; 34 (2): S37-S39. 83 28. Abad- Jorge, A, Cooper, K, Morris, J, et al. Pediatric Nutrition Standards of Care, University of Virginia Health System, Nutrition Management in Oncology. 2010; 112113. 29. Huhmann M., Review of American Society for Parenteral and Enteral Nutrition Clinical Guidelined for Nutrition Support in Cancer Patients: Nutrition Screening and Assessment. Journal for Parenteral and Enteral Nutrition. 2008; 23 (2): 182-188. 30. den Broeder, E, Lippens, R, Tolboom, J, et al. Effects of naso-gastric tube feeding on the nutritional status of children with cancer. Journal of Parenteral and Eternal Nutrition. 1998; 52(7): 494-500. 31. den Broeder E, Lippens R, Hof M, et al. Nasogastric Tube Feeding in Children with Cancer: The Effect of Two Different Formulas on Weight, Body Composition, and serum Protein Concentrations. Journal of Parenteral and Enteral Nutrition. 2000; 24 (6): 351360. 32. Markey T., Nutritional Considerations in Pediatric Oncology. Journal of Oncology Nursing. 2000; 16 (2): 146-151. 33. Bakish J, Bouffet E, Hargrave D, Laperriere N, Rutka J, Tariq N,. Evaluation of Dietetic Intervention in Children with Medulloblastoma or Supratentorial Primitive Neuroectodermal Tumors. Journal of American Cancer Society. 2003; 98 (5): 1014-1020. 34. Aquino VM, Smryl CB, Hagg R, Mchard KM, Prestridge I, Sandler ES. Enteral NutritionalSupport by Gastrostomy Tube in Children with Cancer. Journal of Pediatrics. 1995; 1: 58-62. 35. Azarnoush, Z, Bruno B, Beghin L, et al. Enteral Nutrition: a first option for nutritional support of children following allo-SCT. Bone Marrow Transplantation. 2012; 47: 11911995 36. Garofolo A., Enteral Nutrition During Bone Marrow Transplantation in Patients with Pediatric Cancer: A Prospective Cohort Study. Sao Paulo Medical Journal. 2012; 130 (3): 159-166. 37. Christensen M, Gattuso J, McCormick J. Parenteral Nutrition Associated with Increased Infection Rate in Children with Cancer. Journal of Cancer. 1993; 72 (9): 2732-2738. 38. Cario MS, Spoone S, Sowden L, Bennetts GA, Towne B, Hodder F. Long-term use of indwelling multipurpose silastic catheters in pediatric patients treated with aggressive chemotherapy. Journal of Clinical Oncology. 1986; 4: 784-788. 39. Sacks, G, Enhancing the Response to Parenteral Nutrition in Critical Care. Nutrition in Clinical Practice. 2003; 19: 226-234. 84 40. Broeder E, Lippens R, Hof M, Tolboom J, Sengers R, Berg A, Houdt N, Hofman Z. Nasogastric Tube Feeding in Children with Cancer: The Effect of Two Different Formulas on Weight, Body Composition, and serum Protein Concentrations. Journal of Parenteral and Enteral Nutrition. 2000; 24 (6): 351-360. 41. Fuchs GJ: Enteral Support of the Hospitalized Child. In: Textbook of Pediatric Nutrition, 2nd edition, Suskind RM, Lewinter-Suskind L (eds.). Raven Press, Ltd, New York, 1993: 230-246. 42. Maroun J, Anthony L, Blais N, Burkes R, Dowden S: Prevention and Management of Chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on Chemotherapy-Induced Diarrhea. Current Oncology. 2007; 14 (1): 13-20 43. American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society. 2013; 1-64 44. Devita, Hellman, and Rosenburg’s Cancer 2009: Nutrition Support 45. Locher J, Bonner J, Carroll W, Caudell J, Keith J, Kilgore M: Prophylactic Percutaneous Endoscopic Gastromy Tube Placement in Treatment of Head and Neck Cancer. Journal of Parenteral and Enteral Nutrition. 2011; 35 (3): 365-374 85 Appendix A: Nutritional Assessment: UVAHS Pediatric Nutrition Standards of Care28 1. Food and nutrient intake (1) a) Energy Intake (1.1)- kcal/kg b) Food and beverage intake (1.2) 1) Fluid/beverage intake (1.2.1)- ml/kg 2) Food Intake (1.2.2) c) Breast milk/infant formula intake (1.2.3)-ml/kg, Practice Guideline: note caloric density d) Enteral and parenteral nutrition intake (1.3) 1) Enteral nutrition intake (1.3.1)- kcal/kg, g pro/kg, ml/kg 2) Parenteral nutrition intake (1.3.2)- kcal/kg, g pro/kg, ml/kg, g fat/kg c) Macronutrient intake (1.5) 1) Protein intake (1.5.2)- g/kg d) Micronutrient intake (1.6) 2) Vitamin intake (1.6.1) 3) Mineral/element intake (1.6.2) 2. Food and Nutrient Administration (2) a) Diet history (2.1) 1) Diet order (2.1.1) 2) Enteral and parenteral nutrition administration (2.1.4) 3. Medication and Herbal Supplement Use (3) 4. Medication and herbal supplements (3.1) Knowledge/Beliefs/Attitudes (4) b) Food and nutrition knowledge (4.1) 86 c) Beliefs and attitude (4.2) 5. Behavior (5) a) Adherence (5.1) b) Avoidance behavior (5.2) c) Mealtime behavior (5.4)- Practice Guideline: consider fatigue with feeding, limited number of accepted foods, sensory preferences 6. Factors Affecting Access to Food and Food/Nutrition-Related Supplies (6) d) Food/nutrition program participation (6.1) e) Safe food/meal availability (6.2) 7. Physical Activity and Function a) Breastfeeding (7.1) b) Physical Activity (7.3) 87 Appendix B: Pediatric Oncology Nutrition Data Form 2012-2013: Barriers to Optimal Nutrition Support Name: MRN #: DOB: Sex: Admission Date: Diagnosis: Weight (kg): (___%ile) Weight for length/BMI: (___%ile) PMH: Length (cm): (___%ile) Reason for Admission: PSH: HC (cm): (___%ile) Oncologic Treatments: Data Collection Day 1 Date/Hospital Day Current Weight (kg) 1. Parenteral (Y/N) % Dextrose (order) PRO (g/kg or g/day) 20% IL (mL) Actual Intake: 24 hr Volume (mL/day) Dextrose kcal PRO grams (g/kg)/PRO kcal Lipid kcal Kcal/kg (PN)a 2. Enteral (Y/N) Formula & Density Schedule Route (NG, ND or G-tube) Total Volume (mL/day) Actual Intake: 24 hr Volume (mL/day) Kcal/kg (EN)b TOTAL kcal/kg/d (a+b) 3. Meeting Nutrition Goals (Y/N) Nutrition Goals (kcal/kg, g/kg, ml/kg) % of Goal (kcals, pro) Barriers to Optimal Nutrition # of Days Between RD Recs & Initiation of Nutrition Support Relevant Medications Comments: 88 Day 2 Day 3 Day 4 Day 5 Appendix C: UVA Health System Interview Guide Interview 1: The following questions were used to interview a pediatric oncology nurse practitioner. The purpose of the interview is to obtain the nurse practitioner’s professional opinion regarding pediatric cancer and its nutrition management. 1. What are the most common pediatric cancers treated at UVA Children’s Hospital? The common pediatric cancers are childhood leukemia which accounts for 33% of all pediatric cancers. About 75% of those are acute lymphoblastic leukemia and the other 25% is acute myelogenous leukemia. The ALL requires treatment 2-3 years in treatment and AML requires intense treatment where the children will be admitted for months in the hospital. The children with ALL don’t require TPN although children with AML often require TPN. At UVA there are several CNS brain tumor patients. Often hospitals will place a G-tube concurrent with surgery for port placement or resection. The reason for this is due to the importance of adequate nutrition needed for the children with brain tumors and the challenges they face with nutritional adequacy. The brain tumor patients will often require a G-tube placement during the resection process of the tumor. The lymphomas treatments are close to leukemia although not too intense where they are NPO for long periods of time or require TPN. Some of the leukemia protocols call for high doses of methotrexate which can cause Mucositis which requires IV fluids at night for a week but almost never TPN. Bone tumors (Ewings or Osteo) depends on age but often chemotherapy is very intense where there are infections in , Typhlitis and often require bowel rest with TPN. Soft tissue tumors often can be a challenge with eating especially in toddlers although no Mucositis and almost never g-tube placement. Neuroblastoma patients have long difficult treatments with frequent placement of g-tubes and several rounds of chemotherapy, surgery, and transplant .The neuroblastoma kids have a long difficult road with nutritional management. The rare tumors hepatoblastoma and retinoblastoma don’t require g-tube placement or the use of tpn and usually nutrition is not an issue. However adequate hydration status can present problems. We ask parents to give IVF overnight and kids to provide adequate hydration. A poor appetite is common among these patients and we often prescribe Marinol to stimulate their appetite. Leukemia patients are often on high dose steroids and they “puff up” with an increased appetite usually eating a lot. However the increased appetite doesn’t last very long until it decreases again. 2. Is there a protocol or guideline that you follow regarding the nutritional management of the pediatric oncology patient? If so, please specify the source. We do not have a written guidelines established although we utilize the Children’s Oncology Group recommendations. The Children’s Oncology group consists of hundreds of different institutions that compile their data on treating oncology patients and publish supportive care guidelines that discuss changing treatment or measures to take once a child has lost 5 % of body weight. However we have nothing strict established at UV for our pediatric oncology patients. Other institutions have specific guidelines or protocols for weight loss or caloric intake especially if they have transplant units. 89 3. At what point do you feel nutrition support is indicated in this patient population? What are the factors that influence your decision for starting nutrition support? From the get go the nutrition support is very important. I would consider it just as important as infection control precautions. The child is still growing and having cancer increases those needs for normal growth and development. We try to talk about eating high fiber foods to combat the constipation from chemotherapy treatment. We educate the family on consuming high protein shakes on days the children are not feeling well. We educate the parents on increasing the calories in the foods by adding calorie dense foods during cooking. We are fortunate that we can fall back on enteral nutrition, IVF, or TPN for additional nutritional support. The type of chemotherapy may influence the type of nutritional support because of the symptom management. The different types of chemotherapy treatment may cause mouth sores, nausea, vomiting, the active level of the child, the tumor location. The tumor location can make the nutritional support difficult like Wills tumor and neuroblastoma will affect the stomach. These children often have several surgeries which may affect adequate nutritional intake. . The weight loss will determine enteral nutrition versus parenteral nutrition. However weight loss between 5-10% can determine the imitation of nutritional support. UVA does not have a set number for weight loss as a determinate for nutritional support but we can take into consideration the length of treatment. The maintenance therapy in leukemia with oral chemotherapy has a lower emetic affect and we know the children will gain the weight loss back. However with neuroblastoma or brain tumors the team tends to be more aggressive with nutritional treatment because the nutritional status will worsen throughout the treatment course. 4. What are the advantages and disadvantages to using parenteral nutrition support? We don’t have to “hound” the child to eat because a lot of times the child uses it as a source of control. The nutritional support eliminates the burden of caloric intake and relieves the tension between child and parent. Other advantages include the chemotherapy can cause the loss of electrolytes excreted from the kidneys and this is easily corrected with TPN. We can use the TPN to bolster the child for nutritional adequacy if going they are going to be on a long treatment cycle. If the child hasn’t been eating but previously on TPN then the team will leave the child on the TPN longer. The reasoning is to provide additional nutrition to bolster the child through treatment if we know the next cycle will be tougher to provide additional nutrients. The disadvantages are the TPN has a higher risk of infection due to the high glucose concentration. The TPN can be harder on the child’s liver because of the lipid content and the overall metabolism of the concentration of TPN. Parenteral nutrition is expensive and the labs to follow the TPN daily are an added expense. When patients are discharged to home on TPN this can add to another source of infection. The parents will administer the TPN through a central line. The manipulation of the intravenous line puts the child at higher risk for infection. 5. What are the advantages and disadvantages to using enteral nutrition support (whether through NGT or via GT)? The advantages of the enteral nutrition are the ability to provide oral medication through the tube. It can help to alleviate the tension between the child and parent. The parent will not have to worry about asking child to finish every bite. The enteral nutrition is not as expensive as 90 TPN and easier for the family to administer feeds at home with a pump. Pediasure can be purchased from a store but insurance doesn’t cover the cost of the product. The disadvantages of enteral feeds are that the children hate the NG tubes. The tubing can dislodge during episodes of emesis. The tubing can serve as a source of infection with Mucositis or an irritant. The process of cleaning the tubing can cause issues for the parents. The tubing may increase the anxiety for the parents or children and provide additional stress to the family. The children often associate pain with the g-tube even if pain is due to the tumor. If the g-tube comes out then it requires surgical placement. 6. What are the biggest barriers or complications associated with using nutrition support in the inpatient setting? Please note if you are referring to EN and/or PN. We don’t’ usually have barriers because the parents are usually on board with the idea of nutritional support. However with the g-tubes children are usually hesitant but TPN is the option children prefer. The oncology RD is a good supportive resource for when we are having questions regarding nutritional support. 7. How do you feel nutrition management of the pediatric oncology patient could be improved? We need better handouts to provide to the families and currently we follow a handout made specifically for adult oncology patients. I am in the process of developing handouts to meet UVA standards. A lot of the nutritional management we question in retrospective for example placing a g-tube sooner, different placement of tube, or started TPN earlier. It is always a process of trial and error because it is dependent upon the individual patient. We could improve the nutritional management with the RD rounding with the team in the morning. The patients in outpatient clinic are struggling with their nutritional support. I feel this may improve if they had access to the inpatient RD they are familiar with during their admission. I feel it would be beneficial to have follow-up with inpatients when they arrive for their outpatient clinic. It would be beneficial to have more access to an RD in the outpatient clinic. The nurse practitioners would benefit from knowing the options available for the different enteral products (flavors) via resources provided (handouts).The pediatric nutritional support handbook provided to the staff especially the nurse practitioners and attending’s would be beneficial as well. It would be helpful for the nurse practitioners to receive an in-service from the RD on the nutritional support information and guidelines. 91 Interview 2: The following questions were used to interview a current RN at UVA Children’s Hospital. The purpose of the interview is to obtain the bedside RNs professional opinion regarding use of nutritional support in pediatric cancer patients. 1. What are the advantages and disadvantages to using parenteral nutrition support? When an oncology patient is experiencing Mucositis or nausea, it is nice for them to be provided with parenteral nutrition, so they do not have to have the increased stress of eating enough to maintain proper nutrition. The downfall to getting parenteral nutrition is that when a patient is neutropenic and receiving multiple antibiotics, they are receiving large fluid volumes. So it is very important to accurately record input and output. 2. What are the advantages and disadvantages to using enteral nutrition support (whether through NGT or via GT)? Some of our oncology patients are getting their calories via NGT/GT and they are not eating by mouth. This is hard for some families to understand because they came into the hospital with a child that was eating their meals and now they are refusing to eat. But on the other side it is nice to be able to feed a patient even when they refuse to take all of their calories by mouth. 3. At what point do you feel nutrition support is initiated in this patient population? (ie: after X amount of days not eating, or a certain % weight loss, etc.) I feel that this depends on the patient and which attending is on service. It can also depend on how the parents feel about the lack of PO intake. 4. What are the biggest barriers or complications that you see associated with using nutrition support in the inpatient setting? Please note if you are referring to EN and/or PN. For enteral feedings it is often difficult getting the proper formula. We have to beg and borrow from other units when the store room states they are out of a certain formula. This tends to happen more often than not. I do not feel that there are many barriers with PN. 5. How do you feel nutrition management of the pediatric oncology patient could be improved? I know that every patient and their treatment vary so it would not be feasible to have a standard protocol. I think that tube feeds should be started earlier than they are sometimes. I know that it has to do with parent’s hesitation and wanting to see if the 92 patient’s intake will improve on its own. Overall I feel that the physicians and dieticians are very involved in the management of nutrition for our oncology patients. Interview 3: The following questions were used to interview a current RD at UVA Children’s Hospital. The purpose of the interview is to obtain the RDs professional opinion regarding use of nutritional support in pediatric cancer patients. 1. What are the most common pediatric cancers treated at UVA Children’s Hospital? I’m not sure of the official numbers, but the diagnoses that seem most prevalent include ALL, AML, Wilm’s Tumor, Medulloblastoma, Neuroblastoma, and Sarcomas (osteo, Ewing’s, other) 2. Is there a protocol or guideline that you follow regarding the nutritional management of the pediatric oncology patient? If so, please specify the source. The Children’s Oncology Group has an algorithm for the initiation of nutrition interventions and nutrition support. I typically follow these guidelines with my recommendations. 3. What are the most common topics of nutrition education that are covered with this patient population? We do a lot with maximizing nutritional intake, so using protein and calorie fortifiers, adding dips, sauces, yogurt, cheese, peanut butter to foods, etc. I also work with the families a lot on making the most of the calories the children are drinking, whether they use plain milk, commercial oral nutrition supplements, or their own homemade milkshakes and smoothies. We’ll also talk about how to optimize intake despite severe nausea or vomiting, or any taste changes. Finally, we do talk about general good food safety practices, and if a patient is getting ready for (or has recently had) a bone marrow transplant, then we’ll really hone in on this. 4. At what point do you feel nutrition support is indicated in this patient population? What are the factors that influence your recommendations for starting nutrition support? Based off of the guidelines, if a patient has lost >5% of their UBW, I think it’s worth starting nutrition support. We also take into consideration their upcoming treatments, anticipated side effects, how we think they’ll eat in the interim, overall % wt loss, nutrition status prior to diagnosis to individualize when nutrition support might be indicated. With pediatrics especially, I think it’s important to be proactive vs. reactive to try to ensure the best possible nutrition status, not only to help support and maintain normal growth, but also for improved outcomes related to their response to their treatment regimen. 5. What are the advantages and disadvantages to using parenteral nutrition support? Patients/families seem a lot more comfortable using TPN over EN. I think a lot of this has to do with the fact that the children already have central access, so it seems “easier” and less invasive than a nasogastric or nasojejunal tube. The chemotherapy also often affects the child’s kidney 93 function acutely, and so we see a lot of electrolyte disarray. TPN can be a way to streamline repletion of the electrolytes while still providing the nutrients that the child may be missing out on. Unfortunately, I think that too often we go to TPN as a first line of nutrition support, even if EN may be better suited for the patient. There are significantly greater risks for infection with TPN due to re-accessing their central lines, and the higher dextrose infusion. It’s also less physiologic and does not stimulate the gut mucosa like EN can, and does not provide the immune-stimulating benefits that using the gut can. 6. What are the advantages and disadvantages to using enteral nutrition support (Whether through NGT or via GT)? As previously mentioned using EN is more physiologic and has improved benefits in terms of maintaining the gut mucosa. It’s also substantially less expensive than TPN and has lower risks for infection which is such a big factor for this population, since their immune system is already so compromised. The disadvantages include the fact that a tube needs to be placed, and since it’s typically not a PEG or G-button, which would require a surgery, the child would have to have the NG tubes which are uncomfortable. We also see some issues with self-image due to the placement of the tube. Finally, for a child with active mucositis without prior enteral access, it’s difficult to convince the team to place a tube that might irritate the mucositis or cause bleeding in a child with such low counts. 7. What are the biggest barriers or complications associated with using nutrition support in the inpatient setting? Please note if you are referring to EN and/or PN. I think there are two big issues that fall under that question: 1) barriers to starting nutrition support, and then 2) barriers to optimizing nutrition support. As I’ve previously mentioned, I think there’s a certain stigma patients/families associate with NG tubes which make them reluctant to using them as a part of their care plans. Families often see weight loss as “expected” given the nature of their child’s treatment and IV nutrition can be seen as an “easier” fix. This also can affect quality of life, and with other issues like mucositis and n/v, often EN is not started as proactively as it could be. Once it’s started I think the biggest barriers to optimizing nutrient delivery are 1) tolerance, and 2) feeds being held due to interruptions. 8. How do you feel nutrition management of the pediatric oncology patient could be improved? I think we could be more proactive instead of reactive in starting nutrition support, particularly EN. I also think it would be helpful to have more follow-up from an RD in clinic, to allow for the continuum of care, and to be able to identify needs and adjust regimens in a timelier manner (vs. only seeing these patients when they’re inpatient). 94 Appendix D: Algorithm for Nutritional Intervention in the Pediatric Oncology Patient15 Identify appropriate category: Age > 2 years choose either BMI or IBW Age < 2 years choose WT/LT (Weight for Length) or IBW Underweight Normal th < 5 % ile < 10th % ile < 70% severe > 70-80% Moderate > 80-90% Mild BMI WT/LT IBW Risk of Overweight/Overweight th 5-85 % ile 10-90th % ile > 90-110% >85-95% ile >110-120% > 95th % ile > 90th % ile > 120% > 5 % wt loss from Usual Body Weight or during therapy Or Crossing > 2 % ile channels NO Meeting >80% estimated nutritional needs through oral intake (food and supplements) YES YES NO NO Will impending treatment adversely affect nutritional status and ability to meet needs orally? YES Encourage oral intake/ supplements and monitor weight/ adequacy of diet once a month NO Oncologic prognosis warrants TPN or EN YES Can patient safely tolerate/absorb nutrients via GI tract YES NO Is patient a candidate for EN? NO Is expected need for nutritional support > 5 days? YES NO Can intolerance be alleviated by changing formula or using antiemetic’s/motility agents TPN until GI tract can be safely used YES NO Monitor and intervene as needed YES High risk of pulmonary aspiration or excessive emesis NO TPN YES Gastric feeds Post-pyloric feeds NO EN required for > 3 months NO Can tolerance be alleviated by changing formula, method of administration or adding medication? YES Nasoenteric EN: Nasogastric Nasoduodenal Nasojejunal YES Implement necessary changes NO Provide EN as tolerated and wean when oral consumption is > 50% estimated needs YES 95 Enterostomy EN (PEG vs. G-tube): Gastrostomy Jejunostomy Gastrojejunostomy Can patient tolerate feedings in strength and amounts necessary to meet estimated needs?