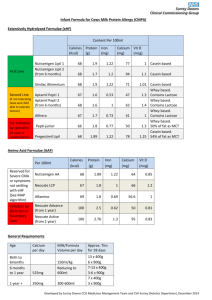
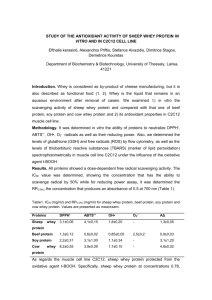
Dairy, Nutrition and Human Health The comparative effects of whey
advertisement

Dairy, Nutrition and Human Health The comparative effects of whey and casein in mildly hypertensive subjects: an 8-wk, randomised, double-blind, cross-over dietary intervention study (the Whey 2 Go study) Á.A. Fekete1,2, D.I. Givens2, S. Todd3, and J.A. Lovegrove1 1 Hugh Sinclair Unit of Human Nutrition and Institute for Cardiovascular and Metabolic Research, Department of Food and Nutritional Sciences University of Reading, Reading, United Kingdom 2 Food Production and Quality Division, Centre for Dairy Research, School of Agriculture, Policy and Development, University of Reading, United Kingdom 3 Department of Mathematics and Statistics, University of Reading, Reading, United Kingdom BACKGROUND: A growing number of epidemiological studies suggest that milk and dairy consumption have a favourable effect on cardiovascular health, and more specifically on blood pressure (BP). Hypertension is the major cause of death worldwide and strategies for its prevention or treatment are urgently needed. Milk proteins may have BP-lowering effects, however the putative mechanism(s) by which whey and casein effect blood pressure and vascular function are unclear. A possible mechanism of action is angiotensin converting enzyme (ACE) inhibition, an important modulator of BP. OBJECTIVE: The aim of this study was to determine the impact of milk proteins on BP and vascular function. We conducted an 8-wk, randomised, double-blinded, cross-over dietary intervention study. Forty-two adults aged 30-70 y with mild hypertension were randomised to consume 56 g whey protein isolates, 56 g Ca-caseinate and 54 g of maltodextrin daily for an 8-week period, separated by 4-week washout periods. The primary outcome measure was 24-h ambulatory BP, secondary outcome measures included office BP and arterial stiffness (assessed by pulse wave analysis (PWA) and digital volume pulse (DVP)). Linear mixed model ANOVA was used to examine effects of the treatments. We also performed an in vitro study to assess the ACE inhibition activity of the whey protein isolate and Ca-caseinate used in the human trial. RESULTS: Thirty-eight participants completed the study, with a mean (SD) age of 52.1 (13) y and BP of 135/84 (14 and 11) mmHg. Product A reduced ambulatory systolic (SBP) (-4.23 mmHg, overall treatment effect p=0.003) and diastolic BP (DBP) (-3.70 mmHg, overall treatment effect p=0.001) compared with Product B (p=0.009 for SBP and p=0.03 for DBP) and Product C (p=0.002 for SBP, p=0.001 for DBP). Product A also significantly reduced office SBP compared to Product C (p=0.0106). We found no significant effects on arterial stiffness markers (PWA or DVP). However there were significant reductions in peripheral and central systolic pressure, assessed by PWA after Product A consumption compared to Product C (peripheral SP p=0.0023, peripheral DP p=0.0328, and central SP p=0.0041, central DP p=0.0325) and Product B (peripheral SP p=0.0226, and central SP p=0.0216). Both whey protein isolate (57.5%) and Ca-casein isolate (10.0%) inhibited ACE in the in vitro study, while maltodextrin showed no inhibition (0%). CONCLUSION: Dietary approaches to reduce BP could prove invaluable, since food-derived proteins have no reported side-effects, in contrast to pharmacological antihypertensive interventions, and could therefore prove to be a safe treatment for individuals with co-morbidities. It is of note that even small reductions in BP at a population level can result in significant decrease in stroke, coronary heart disease and total mortality. Results from this study indicates significant beneficial effects of Product A on BP measures compared with the two other treatments and significant ACE inhibitory effects of the whey and casein proteins. This study will determine the impact of unhydrolysed whey and casein on BP and vascular function and identify potential putative mechanisms of action. This randomised, controlled dietary intervention trial was registered at www.clinicaltrials.gov. Note: The majority of analyses and code braking of treatment products will be completed by Sept 2015.